EIPH - could there be links to sudden death and pulmonary haemorrhage?
Dr Peter W. Physick-Sheard, BVSc, FRCVS, explores preliminary research and hypotheses, being conducted by the University of Guelph, to see if there is a possibility that these conditions are linked and what this could mean for future management and training of thoroughbreds.
"World's Your Oyster,” a three-year-old thoroughbred mare, presented at the veterinary hospital for clinical examination. She won her maiden start as a two-year-old and placed once in two subsequent starts. After training well as a three-year-old, she failed to finish her first start, easing at the top of the stretch, and was observed to fade abruptly during training. Some irregularity was suspected in heart rhythm after exercise. Thorough clinical examination, blood work, ultrasound of the heart and an ECG during rest and workout revealed nothing unusual.
Returning to training, Oyster placed in six of her subsequent eight starts, winning the last two. She subsequently died suddenly during early training as a four-year-old. At post-mortem, diagnoses of pulmonary haemorrhage and exercise-induced pulmonary haemorrhage were established—a very frustrating and unfortunate outcome.
Across the racing world, a case like this probably occurs daily. Anything that can limit a horse's ability to express its genetic potential is a major source of anxiety when training. The possibility of injury and lameness is the greatest concern, but a close second is respiratory disease, with bleeding from the lungs (most often referred to as exercise induced pulmonary [lung] haemorrhage or EIPH) being high on the list.
EIPH is thought to occur in as many as 85 percent of racehorses, and may initially be very mild without obvious clinical consequences. In some cases it can be associated with haemorrhage of sufficient severity for blood to appear at the nostrils, even at first occurrence. In many racing jurisdictions this is a potentially career-ending problem. In these horses, an impact on performance is unquestionable. Bleeding from the lungs is the reason for the existence of ‘Lasix programs,’ involving pre-race administration of a medication considered to reduce haemorrhage. Such programs are controversial—the justifications for their existence ranging from addressing welfare concerns for the horse to dealing with the performance impacts.
Much less frequently encountered is heavy exercise-associated bleeding from the nostrils (referred to as epistaxis), which can sometimes be accompanied by sudden death, during or shortly after exercise. Some horses bleed heavily internally and die without blood appearing at the nostrils. Haemorrhage may only become obvious when the horse is lying on its side, or not until post-mortem. Affected animals do not necessarily have any history of EIPH, either clinically or sub-clinically. There is an additional group of rare cases in which a horse simply dies suddenly, most often very soon after work and even after a winning performance, and in which little to nothing clearly explains the cause on post-mortem. This is despite the fact most racing jurisdictions study sudden death cases very closely.
EIPH is diagnosed most often by bronchoscopy—passing an endoscope into the lung after work and taking a look. In suspected but mild cases, there may not be sufficient haemorrhage to be visible, and a procedure called a bronchoalveolar lavage is performed. The airways are rinsed and fluid is collected and examined microscopically to identify signs of bleeding. Scoping to confirm diagnosis is usually a minimum requirement before a horse can be placed on a Lasix program.
Are EIPH, severe pulmonary haemorrhage and sudden death related? Are they the same or different conditions?
At the University of Guelph, we are working on the hypothesis that most often they are not different—that it’s degrees of the same condition, or closely related conditions perhaps with a common underlying cause. We see varying clinical signs as being essentially a reflection of severity and speed of onset of underlying problems.
Causes in individual cases may reflect multiple factors, so coming at the issues from several different directions, as is the case with the range of ongoing studies, is a good way to go so long as study subjects and cases are comparable and thoroughly documented. However, starting from the hypothesis that these may all represent basically the same clinical condition, we are approaching the problem from a clinical perspective, which is that cardiac dysfunction is the common cause.
Numerous cardiac disorders and cellular mechanisms have the potential to contribute to transient or complete pump (heart) failure. However, identifying them as potential disease candidates does not specifically identify the role they may have played, if any, in a case of heart failure and in lung haemorrhage; it only means that they are potential primary underlying triggers. It isn't possible for us to be right there when a haemorrhage event occurs, so almost invariably we are left looking at the outcome—the event of interest has passed. These concerns influence the approach we are taking.
Background
The superlative performance ability of a horse depends on many physical factors:
Huge ventilatory (ability to move air) and gas exchange capacity
Body structure including limb length and design - allows it to cover ground rapidly with a long stride
Metabolic adaptations - supports a high rate of energy production by burning oxygen, tolerance of severe metabolic disruptions toward the end of race-intensity effort
High cardiovascular capacity - allows the average horse to pump roughly a brimming bathtub of blood every minute
At race intensity effort, these mechanisms, and more, have to work in coordination to support performance. There is likely not much reserve left—two furlongs (400m) from the winning post—even in the best of horses. There are many wild cards, from how the horse is feeling on race day to how the race plays out; and in all horses there will be a ceiling to performance. That ceiling—the factor limiting performance—may differ from horse to horse and even from day to day. There’s no guarantee that in any particular competition circumstances will allow the horse to perform within its own limitations. One of these factors involves the left side of the heart, from which blood is driven around the body to the muscles.
A weak link - filling the left ventricle
The cardiovascular system of the horse exhibits features that help sustain a high cardiac output at peak effort. The feature of concern here is the high exercise pressure in the circulation from the right ventricle, through the lungs to the left ventricle. At intense effort and high heart rates, there is very little time available to fill the left ventricle—sometimes as little as 1/10 of a second; and if the chamber cannot fill properly, it cannot empty properly and cardiac output will fall. The circumstances required to achieve adequate filling include the readiness of the chamber to relax to accept blood—its ‘stiffness.’ Chamber stiffness increases greatly at exercise, and this stiffened chamber must relax rapidly in order to fill. That relaxation seems not to be sufficient on its own in the horse at high heart rates. Increased filling pressure from the circulation draining the lungs is also required. But there is a weak point: the pulmonary capillaries.
These are tiny vessels conducting blood across the lungs from the pulmonary artery to the pulmonary veins. During this transit, all the gas exchange needed to support exercise takes place. The physiology of other species tells us that the trained lung circulation achieves maximum flow (equivalent to cardiac output) by reducing resistance in those small vessels. This process effectively increases lung blood flow reserve by, among other things, dilating small vessels. Effectively, resistance to the flow of blood through the lungs is minimised. We know this occurs in horses as it does in other species; yet in the horse, blood pressure in the lungs still increases dramatically at exercise.
If this increase is not the result of resistance in the small vessels, it must reflect something else, and that appears to be resistance to flow into the left chamber. This means the entire lung circulation is exposed to the same pressures, including the thin-walled capillaries. Capillaries normally work at quite low pressure, but in the exercising horse, they must tolerate very high pressures. They have thin walls and little between them, and the air exchange sacs in the lung. This makes them vulnerable. It's not surprising they sometimes rupture, resulting in lung haemorrhage.
Recent studies identified changes in the structure of small veins through which the blood flows from the capillaries and on toward the left chamber. This was suspected to be a pathology and part of the long-term consequences of EIPH, or perhaps even part of the cause as the changes were first identified in EIPH cases. It could be, however, that remodelling is a normal response to the very high blood flow through the lungs—a way of increasing lung flow reserve, which is an important determinant of maximum rate of aerobic working.
The more lung flow reserve, the more cardiac output and the more aerobic work an animal can perform. The same vein changes have been observed in non-racing horses and horses without any history or signs of bleeding. They may even be an indication that everything is proceeding as required and a predictable consequence of intense aerobic training. On the other hand, they may be an indication in some horses that the rate of exercise blood flow through their lungs is a little more than they can tolerate, necessitating some restructuring. We have lots to learn on this point.
If the capacity to accommodate blood flow through the lungs is critical, and limiting, then anything that further compromises this process is likely to be of major importance. It starts to sound very much as though the horse has a design problem, but we shouldn't rush to judgement. Horses were probably not designed for the very intense and sustained effort we ask of them in a race. Real-world situations that would have driven their evolution would have required a sprint performance (to avoid ambush predators such as lions) or a prolonged slower-paced performance to evade predators such as wolves, with only the unlucky victim being pushed to the limit and not the entire herd.
Lung blood flow and pulmonary oedema
There is another important element to this story. High pressures in the capillaries in the lung will be associated with significant movement of fluid from the capillaries into lung tissue spaces. This movement in fact happens continuously at all levels of effort and throughout the body—it's a normal process. It's the reason the skin on your ankles ‘sticks’ to the underlying structures when you are standing for a long time. So long as you keep moving a little, the lymphatic system will draw away the fluid.
In a diseased lung, tissue fluid accumulation is referred to as pulmonary oedema, and its presence or absence has often been used to help characterise lung pathologies. The lung lymphatic system can be overwhelmed when tissue fluid is produced very rapidly. When a horse experiences sudden heart failure, such as when the supporting structures of a critical valve fail, one result is massive overproduction of lung tissue fluid and appearance of copious amounts of bloody fluid from the nostrils.
The increase in capillary pressure under these conditions is as great as at exercise, but the horse is at rest. So why is there no bloody fluid in the average, normal horse after a race? It’s because this system operates very efficiently at the high respiratory rates found during work: tissue fluid is pumped back into the circulation, and fluid does not accumulate. The fluid is pumped out as quickly as it is formed. An animal’s level of physical activity at the time problems develop can therefore make a profound difference to the clinical signs seen and to the pathology.
Usual events with unusual consequences
If filling the left ventricle and the ability of the lungs to accommodate high flow at exercise are limiting factors, surely this affects all horses. So why do we see such a wide range of clinical pictures, from normal to subclinical haemorrhage to sudden death?
Variation in contributing factors such as type of horse, type and intensity of work, sudden and unanticipated changes in work intensity, level of training in relation to work and the presence of disease states are all variables that could influence when and how clinical signs are seen, but there are other considerations.
Although we talk about heart rate as a fairly stable event, there is in fact quite a lot of variation from beat to beat. This is often referred to as heart rate variability. There has been a lot of work performed on the magnitude of this variability at rest and in response to various short-term disturbances and at light exercise in the horse, but not a lot at maximal exercise. Sustained heart rate can be very high in a strenuously working horse, with beats seeming to follow each other in a very consistent manner, but there is in fact still variation.
Some of this variation is normal and reflects the influence of factors such as respiration. However, other variations in rate can reflect changes in heart rhythm. Still other variations may not seem to change rhythm at all but may instead reflect the way electrical signals are being conducted through the heart.
These may be evident from the ECG but would not appear abnormal on a heart rate monitor or when listening. These variations, whether physiologic (normal) or a reflection of abnormal function, will have a presently, poorly understood influence on blood flow through the lungs and heart—and on cardiac filling. Influences may be minimal at low rates, but what happens at a heart rate over 200 and in an animal working at the limits of its capacity?
Normal electrical activation of the heart follows a pattern that results in an orderly sequence of heart muscle contraction, and that provides optimal emptying of the ventricles. Chamber relaxation complements this process.
An abnormal beat or abnormal interval can compromise filling and/or emptying of the left ventricle, leaving more blood to be discharged in the next cycle and back up through the lungs, raising pulmonary venous pressure. A sequence of abnormal beats can lead to a progressive backup of blood, and there may not be the capacity to hold it—even for one quarter of a second, a whole cardiac cycle at 240 beats per minute.
For a horse that has a history of bleeding and happens to be already functioning at a very marginal level, even minor disturbances in heart rhythm might therefore have an impact. Horses with airway disease or upper airway obstructions, such as roarers, might find themselves in a similar position. An animal that has not bled previously might bleed a little, one that has a history of bleeding may start again, or a chronic bleeder may worsen.
Relatively minor disturbances in cardiac function, therefore, might contribute to or even cause EIPH. If a horse is in relatively tough company or runs a hard race, this may also contribute to the onset or worsening of problems. Simply put, it's never a level playing field if you are running on the edge.
Severe bleeding
It has been suspected for many years that cases of horses dying suddenly at exercise represent sudden-onset cardiac dysfunction—most likely a rhythm disturbance. If the rhythm is disturbed, the closely linked and carefully orchestrated sequence of events that leads to filling of the left ventricle is also disturbed. A disturbance in cardiac electrical conduction would have a similar effect, such as one causing the two sides of the heart to fall out of step, even though the rhythm of the heart may seem normal.
The cases of horses that bleed profusely at exercise and even those that die suddenly without any post-mortem findings can be seen to follow naturally from this chain of events. If the changes in heart rhythm or conduction are sufficient, in some cases to cause massive pulmonary haemorrhage, they may be sufficient in other cases to cause collapse and death even before the horse has time to exhibit epistaxis or even clear evidence of bleeding into the lungs.
EIPH and dying suddenly
If these events are (sometimes) related, why is it that some horses that die of pulmonary haemorrhage with epistaxis do not show evidence of chronic EIPH? This is one of those $40,000 questions. It could be that young horses have had limited opportunity to develop chronic EIPH; it may be that we are wrong and the conditions are entirely unrelated. But it seems more likely that in these cases, the rhythm or conduction disturbance was sufficiently severe and/or rapid in onset to cause a precipitous fall in blood pressure with the animal passing out and dying rapidly.
In this interpretation of events, the missing link is the heart. There is no finite cutoff at which a case ceases to be EIPH and becomes pulmonary haemorrhage. Similarly, there is no distinct point at which any case ceases to be severe EIPH and becomes EAFPH (exercise-associated fatal pulmonary haemorrhage). In truth, there may simply be gradation obscured somewhat by variable definitions and examination protocols and interpretations.
The timing of death
It seems from the above that death should most likely take place during work, and it often does, but not always. It may occur at rest, after exercise. Death ought to occur more often in racing, but it doesn't.
The intensity of effort is only one factor in this hypothesis of acute cardiac or pump failure. We also have to consider factors such as when rhythm disturbances are most likely to occur (during recovery is a favourite time) and death during training is more often a problem than during a race.
A somewhat hidden ingredient in this equation is possibly the animal's level of emotional arousal, which is known to be a risk factor in humans for similar disturbances. There is evidence that emotions/psychological factors might be much more important in horses than previously considered. Going out for a workout might be more stimulating for a racehorse than a race because before a race, there is much more buildup and the horse has more time to adequately warm up psychologically. And then, of course, temperament also needs to be considered. These are yet further reasons that we have a great deal to learn.
Our strategy at the University of Guelph
These problems are something we cannot afford to tolerate, for numerous reasons—from perspectives of welfare and public perception to rider safety and economics. Our aim is to increase our understanding of cardiac contributions by identifying sensitive markers that will enable us to say with confidence whether cardiac dysfunction—basically transient or complete heart failure—has played a role in acute events.
We are also looking for evidence of compromised cardiac function in all horses, from those that appear normal and perform well, through those that experience haemorrhage, to those that die suddenly without apparent cause. Our hope is that we can not only identify horses at risk, but also focus further work on the role of the heart as well as the significance of specific mechanisms. And we hope to better understand possible cardiac contributions to EIPH in the process. This will involve digging deeply into some aspects of cellular function in the heart muscle, the myocardium of the horse, as well as studying ECG features that may provide insight and direction.
Fundraising is underway to generate seed money for matching fund proposals, and grant applications are in preparation for specific, targeted investigations. Our studies complement those being carried out in numerous, different centres around the world and hopefully will fill in further pieces of the puzzle. This is, indeed, a huge jigsaw, but we are proceeding on the basis that you can eat an elephant if you're prepared to process one bite at a time.
How can you help? Funding is an eternal issue. For all the money that is invested in horses there is a surprisingly limited contribution made to research and development—something that is a mainstay of virtually every other industry; and this is an industry.
Look carefully at the opportunities for you to make a contribution to research in your area. Consider supporting studies by making your experience, expertise and horses available for data collection and minimally invasive procedures such as blood sampling.
Connect with the researchers in your area and find out how you can help. Watch your horses closely and contemplate what they might be telling you—it's easy to start believing in ourselves and to stop asking questions. Keep meticulous records of events involving horses in your care— you never know when you may come across something highly significant. And work with researchers (which often includes track practitioners) to make your data available for study.
Remember that veterinarians and university faculty are bound by rules of confidentiality, which means what you tell them should never be ascribed to you or your horses and will only be used without any attribution, anonymously. And when researchers reach out to you to tell you what they have found and to get your reactions, consider actually attending the sessions and participating in the discussion; we can all benefit—especially the ultimate beneficiary which should be the horse. We all have lots to learn from each other, and finding answers to our many challenges is going to have to be a joint venture.
Finally, this article has been written for anybody involved in racing to understand, but covering material such as this for a broad audience is challenging. So, if there are still pieces that you find obscure, reach out for help in interpretation. The answers may be closer than you think!
Oyster
And what about Oyster? Her career was short. Perhaps, had we known precisely what was going on, we might have been able to treat her, or at least withdraw her from racing and avoid a death during work with all the associated dangers—especially to the rider and the associated welfare concerns.
Had we had the tools, we might have been able to confirm that whatever the underlying cause, she had cardiac problems and was perhaps predisposed to an early death during work. With all the other studies going on, and knowing the issue was cardiac, we might have been able to target her assessment to identify specific issues known to predispose.
In the future, greater insight and understanding might allow us to breed away from these issues and to better understand how we might accommodate individual variation among horses in our approaches to selection, preparation and competition. There might be a lot of Oysters out there!
For further information about the work being undertaken by the University of Guelph
Contact - Peter W. Physick-Sheard, BVSc, FRCVS.
Professor Emeritus, Ontario Veterinary College, University of Guelph - pphysick@uoguelph.ca
Research collaborators - Dr Glen Pyle, Professor, Department of Biomedical Sciences, University of Guelph - gpyle@uoguelph.ca
Dr Amanda Avison, PhD Candidate, Department of Biomedical Sciences, University of Guelph. ajowett@uoguelph.ca
References
Caswell, J.I. and Williams K.J. (2015), Respiratory System, In ed. Maxie, M. Grant, 3 vols., 6th edn., Jubb, Kennedy and Palmer’s Pathology of Domestic Animals, 2; London: Elsevier Health Sciences, 490-91.
Hinchcliff, KW, et al. (2015), Exercise induced pulmonary hemorrhage in horses: American College of Veterinary Internal Medicine consensus statement, J Vet Intern Med, 29 (3), 743-58.
Rocchigiani, G, et al. (2022), Pulmonary bleeding in racehorses: A gross, histologic, and ultrastructural comparison of exercise-induced pulmonary hemorrhage and exercise-associated fatal pulmonary hemorrhage, Vet Pathol, 16:3009858221117859. doi: 10.1177/03009858221117859. Online ahead of print.
Manohar, M. and T. E. Goetz (1999), Pulmonary vascular resistance of horses decreases with moderate exercise and remains unchanged as workload is increased to maximal exercise, Equine Vet. J., (Suppl.30), 117-21.
Vitalie, Faoro (2019), Pulmonary Vascular Reserve and Aerobic Exercise Capacity, in Interventional Pulmonology and Pulmonary Hypertension, Kevin, Forton (ed.), (Rijeka: IntechOpen), Ch. 5, 59-69.
Manohar, M. and T. E. Goetz (1999), Pulmonary vascular resistance of horses decreases with moderate exercise and remains unchanged as workload is increased to maximal exercise, Equine Vet. J., (Suppl.30), 117-21.
The Often Overlooked Equine Sacroiliac Joint
Horses that present as sore in the hindquarters can be perplexing to diagnose. Sometimes the problem is found in the last place you look – the sacroiliac joint.
Article by Annie Lambert
Even though the sacroiliac joint (SI) was on veterinary radars long ago, due to its location buried under layers of muscle in the equine pelvic region, the joint and surrounding ligaments were tough to diagnose and treat.
The sacroiliac joint is often a source of lower back discomfort in race and performance horses. Trainers may notice several clinical signs of a problem. These hints include sensitivity to grooming, objections to riders getting legged up, stiffness of motion, pain to manual palpation of the rump or back, resistance to being shod behind and poor performance.
Of course, those symptoms could describe other hind limb soundness issues, making the origin of the problem arduous to ascertain. A thorough physical examination with complete therapeutic options can relieve sacroiliac pain. The treatments are complicated, however, by the anatomy of the SI area.
The equine pelvis is composed of three fused bones: ilium, ischium and pubis. The sacrum, the lower part of the equine back, is composed of five fused vertebrae. The sacroiliac joint is located where the sacrum passes under the top of the pelvis (tubera sacrale). The dorsal, ventral and interosseous sacroiliac ligaments help strengthen the SI joint.
The SI and surrounding ligaments provide support during weight bearing, helping to transfer propulsive forces of the hind limbs to the vertebral column—creating motion much like the thrust needed to break from the starting gate.
Sound complicated? It certainly can be.
Diagnosing Dilemmas
It wasn’t until modern medical technology advanced that the SI could be explored seriously as a cause of hind lameness.
“The sacroiliac is one of the areas that’s very hard to diagnose or image,” explained Dr. Michael Manno, a senior partner of San Dieguito Equine Group in San Marcos, California. “[Diagnostics] of the area probably correlated with bone scans or nuclear scintigraphy. You can’t really use radiographs because the horse is so massive and there is so much muscle, you can’t get a good image.
“About the only time you can focus on the pelvis and get a decent radiograph is if the horse is anesthetized—you have a big [x-ray] machine and could lay the horse down. But, it’s hard because with anything close to a pelvic injury, the last thing you want to do is lay them down and have them have to get back up.”
The nuclear scintigraphs give a good image of hip, pelvis and other anatomical structures buried deep in the equine body, according to Manno, a racetrack practitioner. “Those images can show areas of inflammation that could pretty much be linked right to the SI joint.”
The other modern technological workhorse in the veterinary toolbox is the digital ultrasound machine. Manno pointed out that veterinarians improved diagnostics as they improved their ultrasounding skills and used those skills to ultrasound areas of the body they never thought about before. Using different techniques, frequencies and various heads on the machine’s probe, the results can be fairly remarkable.
“The ultrasound showed you could really image deeper areas of the body, including an image of the sacroiliac joint,” Manno said. “It can also show some ligament issues.”
Where the SI is buried under the highest point of a horse’s rump, and under heavy gluteal muscles, there are two sets of ligaments that may sustain damage and cause pain. The dorsal sacroiliac ligaments do not affect the sacroiliac joint directly, but help secure the ilium to the sacral spine. The ventral sacroiliac ligaments lie deeper, in the sacroiliac joint area, which they help stabilize. These hold the pelvis tight against its spine. The joint itself, being well secured by these ligaments, has little independent movement and therefore contains only minimal joint fluid.
Diagnosing the SI can be complex because horses often travel their normal gait with no change from normal motion—no signs of soreness. Other horses, however, are sore on one leg or another to varying degrees, sometimes with a perceptible limp.
“I don’t know that there is a specific motion,” Manno explained. “You just know that you have a hind end lameness, and I think a lot of performance horses have mildly affected SI joints.
“The horses that are really severe become acutely lame behind, very distinct. You go through the basic diagnostics, and I think most of these horses will show you similar signs as other issues behind. We palpate along the muscles on either side of their spine and they are sore, or you palpate over their croup and you can get them to drop down—that kind of thing. Other times you do an upper limb flexion on them and they might travel weird on the opposite leg. So, it can be a little confusing.”
In the years prior to the early 2000s, the anatomical location of the SI hindered a definite diagnosis; decisions on hind soreness were more of a shrug, “time and rest” treatment evaluation. As one old-time practitioner called it, a SWAG – “Scientific Wild Ass Guess.”
Even with modern tools, making a conclusive diagnosis can be opaque.
“The less affected horses, through exercise and with medications like Robaxin [muscle relaxer] or mild anti-inflammatories, seem to be able to continue to perform,” Manno said. “I don’t know how you can be perfectly sure of an inside joint unless you try to treat it and get results.”
“That’s why bone scans came into play and are really helpful,” Manno added. “You can image that [SI] area from different angles with the machine right over the path of the pelvis, looking down on it or an angle view into it, and then you see it from the side and the back very often. We can get an idea from the different views and angles of where the inflammation is and pinpoint the problem from that.”
Once Manno has a generalized idea of where the problem is, he fine-tunes his hypothesis using more diagnostics with a digital ultrasound machine.
“You can ultrasound from up above and see the joint that way,” he said. “As ultrasound has progressed, we’ve found that the rectal probes the breeding vets have used can also be tuned in to start looking for other things. If you turn them upwards, you can look at the bottom of the pelvis and the SI joint. You can see things through the rectum by just looking straight up. That is a whole new thing that we probably never thought about doing. I don’t profess to be very great at it; it’s not something I do a lot, but there are people that are just wonderful at it.”
Treating a Theorem
But, if the diagnosis is incorrect, the prescribed treatment may be anything but helpful.
“In many cases, if a horse is really sore, you need to be very careful,” cautioned Manno. “What you don’t want to do is go from a strain or some sort of soft tissue injury into a pelvic fracture by trying to keep them going. In many cases you are back in the old rest and time type of treatment.”
Manno pointed out one treatment that has advanced over many years is injecting the SI joint directly. There are a couple of techniques used when injecting the SI. With a blind injection the practitioner directs a long, straight needle into the joint by relying solely on equine anatomy. The other technique employs an ultrasound machine to guide the placement of the needle into the joint.
“Normally we are just injecting cortisone in those cases,” Manno noted. “We are trying to get the inflammatory response to settle down. Hopefully that gives the horse some relief so that they’re a bit more relaxed in their musculature. You know how it is when you get a sore back; it’s hard to keep yourself from cramping, which makes everything worse.”
A slight tweak of that technique is to use a curved needle. When you are positioning the curved needle, it follows the curve of the horse’s anatomy and helps the practitioner direct the injection into the joint.
“It curves right into position for you; it gives you a little help,” Manno confirmed of the curved needle. “Some people are really good with that technique; others still like to go to the straight needle. [The curved needle] helps you approach the site without interference from the bones in that area.”
SI joint injuries affect most performance horses, including Standardbred trotters and pacers, Western performance athletes as well as hunters, jumpers and dressage horses.
The older show horses are often diagnosed with chronic SI pain, sometimes complicated by arthritis. These chronic cases—and admittedly some racehorses—are treated with different therapies. These conservative, nonsurgical treatments have been proven effective.
In addition to stall rest and anti-inflammatories, physical training programs can be useful in tightening the equine patient’s core and developing the topline muscles toward warding off SI pain. Manno, a polo player who also treats polo ponies, believes the hard-working ponies avoid having many SI injuries due to their fitness levels.
“I think these polo horses are similar to a cross between a racehorse and a cutting horse,” Manno opined. “They are running distances and slide stopping and turning.”
Other treatments utilized include shockwave, chiropractic, acupuncture, therapeutic laser and pulsed electromagnetic therapy.
Superior Science
With the new diagnostic tools and advanced protocols in their use, veterinarians can pinpoint the SI joint and surrounding areas much closer. This gives them an improved indication that there definitely is an issue with the sacroiliac.
When there is a question about what is causing hind end lameness, most practitioners begin with blocking from the ground up.
“In many cases with hind end lameness that we can’t figure out, we block the lower leg; if it doesn’t block out down low, we conclude the problem is up high,” Manno said. “Once you get up to the hock you’re out of options of what you can figure out. You start shooting some x-rays, but by the time you get to the stifle, you’re limited. Bone scans and ultrasounds have certainly helped us with diagnosing.”
Manno doesn’t see a lot of SI joint injuries in his practice, but he noted there were cases every now and again. He also opined that there were probably other cases that come up in racehorses on a short-term basis. He also noted that, although it may not be a real prominent injury, that’s not to say it has not gone undiagnosed.
“I think we realize, in many of the horses we treat, that the SI joint is something that may have been overlooked in the past,” Manno concluded. “We just didn’t have the ability to get any firm diagnosis in that area.”
Racetrack Fracture Support Equipment - coming to North America this summer
Words - Ian Wright
Over the last six months, British racecourses have taken a major step forward in racehorse welfare by being provided with fracture support systems (Figure 1). These consist of two sizes of compression boots and flexion splints, both for use in the forelimbs; and a set of modular adjustable splints. Together, these provide appropriate rigid external support for the vast majority of limb fractures that occur during racing. The general principles are that management of all fractures is optimized by applying rapid and appropriate support to provide stability, reduce pain and relieve anxiety.
Figure 1
The fracture support systems are about to make their debut in North America with trials due to take place this summer and fall with the support of the National HBPA.
The fracture support system is provided in two mobile impact resistant carrying boxes that protect the equipment and allow it to be checked before racing. All boots and splints are permanently labeled with individual racecourse identification to ensure return of equipment if it leaves the racetrack.
In the last 25 years there have been major improvements in fracture treatment due to significant advances in surgical techniques (particularly with internal fixation), minimally invasive approaches (arthroscopy) and the use of computed tomography (CT). Arthroscopy and CT allow accurate mapping and alignment of fractures, which is important for all and critical for athletic soundness. All have contributed to improvements in survival rates; and it is now safe to say that with correct care, the vast majority of horses that sustain fractures in racing can be saved. Equally importantly, many can also return to full athletic function including racing.
Fracture incidences and locations vary geographically and are influenced by race types, track surfaces and conditions. There is good evidence that the majority of non-fall related fractures, i.e. those occurring in flat racing and between obstacles in jump racing, are caused by bone fatigue. This is determined by the absolute loads applied to a bone, their speed/frequency and the direction of force application. As seen with stress or fatigue failure in other high-performance working materials in which applied forces are relatively consistent, fractures in racehorse bones occur at common sites, in particular configurations, and follow similar courses. Once the fracture location has been identified, means of counteracting forces which distract (separate) the bone parts can therefore be reliably predicted and countered.
Worldwide, the single most common racing fracture is that of the metacarpal/metatarsal condyles (condylar fracture). In Europe, the second most common fracture is a sagittal/parasagittal fracture of the proximal phalanx (split pastern). Both are most frequent in the forelimbs.
In the United States, particularly when racing on dirt, mid-body fractures of both proximal sesamoid bones, which destabilize the fetlock (almost always in the forelimbs), are the most common reason for on-course euthanasia. They occur less frequently when racing on turf.
There is no specific data documenting outcomes of horses that have sustained fractures on racecourses. However, there is solid data for the two most common racing injuries. The figures below are a meta-analysis of published data worldwide.
CONDYLAR FRACTURES
Repaired incomplete fractures: 80% returned to racing
Complete non-displaced fractures: 66% of repaired fractures returned to racing
Displaced fractures: 51% raced following repair
Propagating fractures: 40% raced following repair
SPLIT PASTERN
Short incomplete fractures: 65% returned to racing
Long incomplete fractures: 61% returned to racing
Complete fractures: 51% returned to racing
Comminuted fractures in most circumstances end racing careers but with appropriate support and surgical repair, many horses can be saved. There is only one comprehensive series of 64 cases in the literature of which 45 (70%) of treated cases survived.
MID BODY SESAMOID FRACTURES
Uni-axial (single) fractures: 53% raced following screw repair
Bi-axial (both) fractures are career ending but can be salvaged with appropriate emergency support and arthrodesis (fusion) of the fetlock joint. Results of a single series of 52 cases are available in which 65% of horses were able to have unrestricted activity post-operatively primarily as breeding animals
The science behind the development of the fracture support systems comes from two directions. The first is data collected from racecourse injuries and the second, improved understanding of fracture courses and behavior. Data collected from UK flat racecourses between 2000 and 2013 demonstrated that 66% of fractures occurred in the lower limb (from knee and hock down) and of that over 50% of fractures involved the fetlock joints. Condylar fractures are most common, representing 27% of all reported fractures; and of these, approximately two thirds occurred in the forelimbs. Split pasterns were the second most common, accounting for 19% of all fractures with three quarters of these occurring in the forelimbs. These fractures have predictable planes and courses, which means that once recognized, they can effectively be immobilized in a standard manner that is optimal for each fracture type. For condylar fractures and split pasterns, this principally involves extension of the fetlock joint. By contrast, in order to preserve soft tissues and blood supply to the lower limb, fractures of the sesamoid bones require fetlock flexion.
Figure 2
Figure 3
The compression boot is readily applied “trackside” and can be used to stabilize most distal forelimb fractures sufficiently for horses to be humanly moved off the course. It is the temporary immobilization of choice for forelimb condylar fractures and split pasterns (Figure 3). Radiographs can be taken with the boot in place (Figure 4), and this can be maintained for transport. The boot is a rigid construct of fiberglass made from a single mold. The divided front portion is contiguous with a foot plate on which the back of the boot is hinged. Two sizes are available with internal foot widths of 135 and 160mm (5–6 inches). Removable foot inserts are also provided to make minor adjustments for hoof size. The boot is lined with foam rubber and has a rubber sole plate that protects the shell and provides a cushion grip for the foot. When the boot is opened, the injured limb is placed into the front of the boot while the back is closed and secured by sequential adjustment of ski boot clips. When the boot edges are apposed (it cannot be over tightened), immobilization is secure. It is made with a fixed fetlock angle of 150o which counteracts distracting forces and allows horses to weight-bear and load the limb to walk.
Figure 4
Flexion splints (Figure 5) are critical for the survival of horses with breakdown injuries such as sesamoid fractures. They are also suitable for other lower limb injuries, which are supported by fetlock and pastern flexion such as tendon and suspensory ligament injuries and lacerations. The splints are made of aluminum alloy with a secure work-hardened foot plate and conjoined compressed foam-lined front splint, which is angled 30o at the level of the coffin joint and extends to the top of the cannon. Here, there is a shallow foam-covered concavity in which the upper cannon sits, allowing the horse to lean into the splint and load the leg while flexed. The splint is secured to the leg with nylon and Velcro straps. Splints are provided with internal foot widths of 135 and 160mm (5–6 inches) to accommodate variations in horse/hoof sizes.
The modular adjustable splints (Figure 6) are made from heat-treated aluminum alloy. They are lightweight and can be configured to fit the individual horse and regional needs. The splints are 38x19mm (1.5x0.75in) rectangular tubes with an enclosed locking screw I beam. They are light but rigid and secure and are tolerated well. In the hindlimb, the reciprocal apparatus which combines stifle, hock and fetlock joint positions precludes use of a compression boot. However, modular splints provide rigid support for condylar fractures and split pasterns in hindlimbs and are secured—over a bandage to create a parallel sided tube—on the inside and outside of the limb. The splints can also be adjusted and assembled to splint fractures that occur above the fetlock (Figure 7).
Figure 7
Appropriate immobilization effectively stops fracture progression (i.e., getting worse) which not only improves the horse's prospects for recovery but also provides effective relief from pain and anxiety. As flight animals, loss of limb control or function is a major contributor to stress. The relief provided by effective immobilization is substantially greater than provided by any pain killer or sedative. It is also recognized that when fractures occur in the high-adrenaline environment of racing, horses exhibit latent pain syndrome. Application of appropriate rigid support at this time (i.e., on the track) limits pain generation and allows humane movement for considered evaluation, X-ray, etc., away from the racetrack.
In the UK, techniques for application of the boots and splints are taught to racetrack veterinary surgeons at annual seminars run by the Association of Racecourse Veterinary Surgeons (ARVS). The Racecourse Association (RCA) has provided forms to record use and to collect data centrally which, in the fullness of time, will determine impact and help to guide future welfare strategies.
Providing modern, scientifically rational equipment to racecourses has done two things in the UK. First, injured horses are optimally cared for immediately and secondly, it sends out a strong positive public relations message that people involved in racing care. The initiative has been widely welcomed by the British racing industry. “This new equipment will provide the best possible chance for an injury to be properly assessed while discomfort to the horse is significantly reduced and give the best chance of future rehabilitation” Caroline Davies, RCA (Racecourse Association) - Racecourse Services Director.
“The fracture support [system] kit is a major advance in the treatment of horses on the racetrack. It allows immediate effective support to be applied to an injured horse, resulting in pain control and stability, facilitating safe transport from the racecourse to a center of excellence without risk of exacerbating the injury. This will optimize the chance of horses to return to athletic function. This innovation must be seen as a major step forward in horse welfare for the participants in racing and all other equine disciplines.” Simon Knapp, Horse Welfare Board.
What's that noise? An overview of exercise-induced upper airway disorders
by Kate Allen and Geoffrey Lane
The majority of upper airway (‘wind’) disorders affect the regions of the pharynx and larynx. Most of these conditions are only present during exercise, when the upper airway is exposed to large changes in pressures associated with increased breathing rate and effort. This is the reason why performing endoscopy at rest may not give an accurate diagnosis. Endoscopy during strenuous exercise (overground endoscopy) has become key for veterinary surgeons to be able to give an accurate interpretation of upper airway function.
There are many different forms of upper airway disorders. They occur when part of the pharynx or larynx collapses into the airway, causing an obstruction to airflow. This obstruction causes turbulence to airflow, which in turn creates the abnormal noise. Observations of upper airway function during exercise enable veterinary surgeons to estimate the impact of the abnormalities with respect to race performance. Generally speaking, the more the structure collapses and the more the airway is narrowed, the greater the detrimental effect to performance. The mechanisms by which upper airway disorders affect performance are surprisingly complex, but in brief they influence the amount of air the horse can breathe in and also how hard the horse has to work to get that air into the lungs.
A full understanding of an individual horse’s upper airway function allows targeted treatments to be performed. Although the more common treatments have been included here for completeness, it is important for you to discuss individual horses with your own veterinary surgeon.
Understanding the anatomy is the first step to interpreting upper airway function during exercise. When looking at an endoscopic image, the left side of the horse is on the right side of the image as we look at it, and vice versa (figure 1).
Figure 1: Most disorders of the upper airway are named according to the structure that is collapsing. Therefore, understanding the anatomy of the airway will help to understand the individual conditions.
← Horse’s RIGHT side : Horse’s LEFT side →
Fig 2a
Fig 2b
With good upper airway function, we are looking for full abduction (which means opening) of the arytenoid cartilages while the vocal cords and aryepiglottic folds remain stable, and the epiglottis retains a curved shape; the soft palate and pharyngeal walls also remain stable. This gives a wide opening called the rima glottidis for air to enter the lungs (Figure 2 a, b, c).
Figure 2 a, b, Images showing good upper airway function.
Palatal instability and dorsal displacement of the soft palate
In the normal horse, the soft palate is positioned beneath the epiglottis. Palatal instability comprises billowing movement of the soft palate and often coincides with flattening of the shape of the epiglottis. The appearance of palatal instability can differ between horses (Figure 3 a, b, c). Palatal instability often causes an inspiratory noise.
Fig 3a
Fig 3b
Fig 3c
Figure 3 a, b, c: Images showing different types of palatal instability.
Dorsal displacement of the soft palate (DDSP) occurs when the free border of the soft palate becomes displaced and comes to lie above the epiglottis (Figure 4 a, b, c). In this displaced position, there is a substantial obstruction of the rima glottidis. Sudden onset ‘gurgling’ expiratory noises are characteristic of DDSP. Palatal instability almost invariably precedes DDSP, and it is thought these conditions may arise through weakness of the muscles within the palate itself.
Fig 4a
Fig 4b
Figure 4 a, b : Images showing dorsal displacement of the soft palate (DDSP). The epiglottis is no longer visible as the soft palate is now positioned on top of it.
Thus, in younger racehorses, palatal instability and DDSP will often improve with fitness and maturity. In the UK, the two most commonly performed surgical treatments are soft palate cautery and laryngeal tie-forward. The purpose of the soft palate cautery is to induce scar tissue to tighten the soft palate. The tie-forward has a different rationale. In some horses, the larynx slips backward just prior to DDSP, therefore the tie-forward holds the larynx in a more forward position, thereby inhibiting displacement.
Arytenoid cartilage collapse
This condition is also called recurrent laryngeal neuropathy, laryngeal hemiplegia or laryngeal paralysis because it is caused by nerve damage to the muscles of the larynx. During exercise, we observe collapse of the arytenoid cartilage almost always on the left side. In the context of sales, most trainers are familiar with laryngeal function grading applied during resting endoscopy. The purpose of this is to predict what is likely to happen to arytenoid function during exercise. During exercise, arytenoid function is typically graded as A, B or C where A is full abduction, B is partial collapse and C is complete collapse (Figure 5 a, b, c). The majority of horses with grade 1 or 2 laryngeal function at rest have grade A function during exercise (96% and 88% respectively). Arytenoid cartilage collapse causes a harsh inspiratory noise, often termed ‘roaring’.
Fig 5a
Fig 5b
Fig 5c
Figure 5 a, b, c: Images from 3 different racehorses, showing the variations in position of the left arytenoid. The first image shows a good position, followed by horses with increasing severity of collapse. In the last image, there is virtually no opening remaining for airflow.
Arytenoid cartilage collapse occurs when the nerve supply to the left side of the larynx is damaged. The most frequent surgery to improve complete collapse is a ‘tie-back’, which fixes the collapsing left side into a semi-open position. The potential limitation of this surgery is that if the arytenoid is fixed open, it cannot close to protect the rima glottidis during swallowing. Therefore, horses that have had a tie-back are susceptible to inhaling food into the lower airways leading to coughing. The tie-back is associated with a higher risk of complications than all other upper airway surgeries. More recently a nerve grafting surgery has been developed in which a normal local nerve is detached from a local muscle and then implanted into the laryngeal muscles. This avoids the potential complications of food inhalation but does take a few months to take effect. Both of these surgeries can be combined with ‘Hobday’ surgery.
Arytenoid Subluxation
This condition seems to be observed with increasing frequency. We see it most commonly in young flat racehorses; it is far less common in National Hunt horses, which probably reflects maturity of the laryngeal structures. One arytenoid subluxates or slips underneath the other arytenoid (Figure 6 a and b). The full name for this condition is ventromedial luxation of the apex of the corniculate process of the arytenoid cartilage (VLACPA). This condition appears to lead to instability of several other areas of the larynx, most commonly the vocal cords and aryepiglottic folds (Figure 7 a and b). There is limited scientific evidence for the best way to manage this disorder, and at present there is no effective surgical treatment. The instability within the larynx can be exacerbated the more the horse is exercised, therefore limiting the intensity of training to allow the larynx to mature may be recommended.
Fig 6a
Fig 6b
Figure 6 a and b: Images to show a closeup of the arytenoid cartilages. The image on the left is normal, and the two arytenoid cartilages meet in the middle. The image on the right shows that one side of the larynx has subluxated or slipped underneath the other side.
Fig 7a
Fig 7b
Figure 7 and b: Images to show arytenoid subluxation which has led to aryepiglottic fold collapse and vocal cord collapse.
Vocal cord collapse
Vocal cord collapse is often described as mild, moderate or severe, and typically causes a high-pitched inspiratory ‘whistle’ noise. Vocal cord collapse will almost always occur if arytenoid cartilage collapse occurs (Figure 8) but can also occur without arytenoid cartilage collapse (Figure 9). The traditional treatment for vocal cord collapse is the ‘Hobday’ procedure, which aims to remove the mucosal pocket to the side of the vocal cord along with the cord itself.
Figure 8: Image showing left arytenoid cartilage collapse with vocal cord collapse.
Figure 9: Image showing severe bilateral vocal cord collapse.
Aryepiglottic fold collapse
Aryepiglottic fold collapse is when the folds of tissue on the side of the larynx get sucked into the airway (Figure 10 a , b, c). This condition also causes a high-pitched inspiratory noise. It is typically graded as mild, moderate and severe. It most often occurs in conjunction with other conditions that alter the normal conformation of the arytenoid or epiglottis (i.e., palatal instability, arytenoid subluxation, arytenoid cartilage collapse). Treatment aims to remove a section of the folds.
Fig 10a
Fig 10b
Fig 10c
Figure 10 a, b, c: Images showing aryepiglottic fold collapse.
Pharyngeal wall collapse
Pharyngeal wall collapse is when the roof or sides of the pharynx collapse, which tends to obscure the larynx from clear view (Figure 11 a and b). It occurs more commonly in sport horses than racehorses due to head and neck position; the more flexed the head and neck position, the harder it is for the walls to remain stable. The time that we most often observe it in racehorses is at the start of the gallops if they are restrained, and often it will improve as the horse is able to extend its head and neck. This condition also causes a coarse inspiratory noise.
Fig 11a
Fig 11b
Figure 11 a and b: Images showing pharyngeal wall collapse.
Epiglottic entrapment
Although included here for completeness, epiglottic entrapment can usually be diagnosed during a resting endoscopic examination, particularly if the horse is triggered to swallow. The epiglottis becomes enveloped in the excess tissue that should lie underneath it (Figure 12 a and b). Sometimes the epiglottis remains entrapped, but sometimes it will entrap and release on its own which can make the diagnosis more difficult. The noise caused by epiglottic entrapment can vary, depending on the thickness of the entrapping tissue and whether DDSP occurs concurrently. Treatment involves releasing or resecting the excessive tissue.
Fig 12a
Fig 12b
Figure 12 a and b: Images showing epiglottic entrapment in two different horses. The image on the right shows an epiglottic entrapment that is more long standing, and the tissue has become swollen and ulcerated.
The disorders outlined above are described as if they are isolated single entities, but it is commonplace for horses to sustain complex collapse, which means collapse of multiple structures at the same time. Other less common disorders are epiglottic retroversion (when the epiglottis flips up to cover the rima glottidis), and cricotracheal membrane collapse (when there is collapse between the larynx and the trachea). On occasion obstructions to breathing can also occur in the nasal passages and the trachea (i.e., masses, ethmoid haematoma, sinusitis), but are far less common than those of the pharynx and larynx.
Looking forward it is unlikely that any new conditions remain to be discovered. Research now centres around better understanding of the causes of these disorders and how best to prevent and treat them. A particular area of investigation amongst several research groups is understanding how to train the upper airway muscles more appropriately to reduce the prevalence of these disorders and to investigate methods to strengthen the muscles. This would have the potential to reduce the number of horses needing surgical treatments.
Antimicrobials in an age of resistance
By Jennifer Davis and Celia Marr
Growing numbers of bacterial and viral infections are resistant to antimicrobial drugs, but no new classes of antibiotics have come on the market for more than 25 years. Antimicrobial-resistant bacteria cause at least 700,000 human deaths per year according to the World Health Organization (WHO). Equivalent figures for horses are not available, but where once equine vets would have very rarely encountered antimicrobial-resistant bacteria, in recent years this serious problem is a weekly, if not daily, challenge.
The WHO has for several years now, designated a World Antibiotic Awareness Week each November and joining this effort, British Equine Veterinary Association and its Equine Veterinary Journal put together a group of articles exploring this problem in horses.
For more information: https://beva.onlinelibrary.wiley.com/hub/journal/20423306/homepage/sc_antimicrobials_in_an_age_of_resistance
How do bacterial populations develop resistance?
Certain types of bacteria are naturally resistant to specific antimicrobials and susceptible to others. Bacteria can develop resistance to antimicrobials in three ways: bacteria, viruses and other microbes, which can develop resistance through genetic mutations or by one species acquiring resistance from another. Widespread antibiotic use has made more bacteria resistant through evolutionary pressure—the “survival of the fittest” principle means that every time antimicrobials are used, susceptible microbes may be killed; but there is a chance that a resistant strain survives the exposure and continues to live and expand. The more antimicrobials are used, the more pressure there is for resistance to develop.
The veterinary field remains a relatively minor contributor to the development of antimicrobial resistance. However, the risk of antimicrobial-resistant determinants traveling between bacteria, animals and humans through the food chain, direct contact and environmental contamination has made the issue of judicious antimicrobial use in the veterinary field important for safeguarding human health. Putting that aside, it is also critical for equine vets, owners and trainers to recognize we need to take action now to limit the increase of antimicrobials directly relevant to horse health.
How does antimicrobial resistance impact horse health?
Fig 1. This mare’s problems began with colic; she underwent surgery to correct a colon torsion (twisted gut). When the gut wall was damaged, bacteria easily spread throughout the body. The mare developed an infection in her surgical incision and in her jugular veins, progressing eventually to uncontrollable infection—resistant to all available antimicrobials with infection of the heart and lungs.
The most significant threat to both human and equine populations is multidrug-resistant (MDR) pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum beta-lactamase (ESBL) producing Escherichia coli, MDR Klebsiella pneumoniae, Pseudomonas aeruginosa, Enterococcus faecium, and rising MDR strains of Salmonella spp. and Clostridium difficile. In an analysis of 12,695 antibiograms collected from horses in France between 2012-2016, the highest proportion (22.5%) of MDR isolates were S. aureus. Identification of ESBL E.coli strains that are resistant to all available antimicrobial classes has increased markedly in horses. In a sampling of healthy adult horses at 41 premises in France in 2015, 44% of the horses shed MDR E.coli, and 29% of premises shedding ESBL isolates were found in one third of the equestrian premises. Resistant E. coli strains are also being found in post-surgical patients with increasing frequency.
Fig 2. Rhodococcus equi is a major cause of illness in young foals. It leads to pneumonia and lung abscesses, which in this example has spread through the entire lung. Research from Kentucky shows that antimicrobial resistance is increasingly common in this bacterial species.
Of major concern to stud owners, antimicrobial-resistant strains of Rhodococcus equi have been identified in Kentucky in the last decade, and this bacteria can cause devastating pneumonia in foals. Foals that are affected by the resistant strains are unlikely to survive the illness. One of the leading authorities on R.equi pneumonia, Dr. Monica Venner has published several studies showing that foals can recover from small pulmonary abscesses just as quickly without antibiotics, and has pioneered an “identify and monitor” approach rather than “identify and treat.” Venner encourages vets to use ultrasonography to quantify the infected areas within the lung and to use repeat scans, careful clinical monitoring and laboratory tests to monitor recovery. Antimicrobials are still used in foals, which are more severely affected, but this targeted approach helps minimize drug use.
What can we do to reduce the risk of antimicrobial resistance?
The simple answer is stop using antimicrobials in most circumstances except where this is absolutely avoidable. In training yards, antimicrobials are being over-used for coughing horses. Many cases are due to viral infection, for which antibiotics will have little effect. There is also a tendency for trainers to reach for antibiotics rather than focusing on improving air quality and reducing exposure to dust. Many coughing horses will recover without antibiotics, given time. Although it has not yet been evaluated scientifically, adopting the identify and monitor approach, which is very successful in younger foals, might well translate to horses in training in order to reduce overuse of antimicrobials.
Fig 3. Faced with a coughing horse, trainers will often pressure their vet to administer antibiotics, hoping this will clear up the problem quickly. Many respiratory cases will recover without antibiotics, given rest and good ventilation
Vets are also encouraged to choose antibiotics more carefully, using laboratory results to select the drug that will target specific bacteria most effectively. The World Health Organization has identified five classes of antimicrobials as being critically important, and therefore reserved, antimicrobials in human medicine. The critically important antimicrobials which are used in horses are the cephalosporins (e.g., ceftiofur) and quinolones (e.g., enrofloxacin), and the macrolides, which are mainly used in foals for Rhodococcal pneumonia. WHO and other policymakers and opinion leaders have been urging vets and animal owners to reduce their use of critically important antimicrobials for well over a decade now. Critically important antimicrobials should only be used where there is no alternative, where the disease being treated has serious consequences and where there is laboratory evidence to back up the selection. The British Equine Veterinary Association has produced helpful guidelines and a toolkit, PROTECT-ME, to help equine vets achieve this.
How well are we addressing this problem?
Disappointingly, in a recent review of prescribing behavior of three “reserved” antimicrobials at first-opinion equine practices in the USA and Canada between 2006-2012 published in Equine Veterinary Journal, only 5% of prescriptions for the reserved antimicrobials enrofloxacin, ceftiofur and clarithromycin were informed by culture and sensitivity testing. There was also an overall trend of increased prescribing of enrofloxacin across the study period, and despite increasing awareness of the challenge of antimicrobial resistance, a decreasing proportion of enrofloxacin prescriptions were based on culture and sensitivity results.
Judicious use of antimicrobials for surgical patients
Antimicrobials are commonly used in the perioperative period. In both human and veterinary medicine, antimicrobial use for surgical prophylaxis has been a target for reducing or eliminating inappropriate antimicrobial administration. The British Equine Veterinary Association recommends administration of penicillin pre- and post-operatively for 24 hours for clean surgeries; penicillin and gentamicin pre- and post-operatively for five days for contaminated surgeries; and penicillin and gentamicin pre- and post-operatively for 10 days for complicated surgeries. Furthermore, for uncomplicated contaminated wounds (e.g., hoof abscesses), antimicrobial therapy is not recommended. A 2018 survey of perioperative antimicrobial use among equine practitioners in Australia revealed that most equine vets selected an appropriate antimicrobial agent. However, the dose of penicillin chosen was often suboptimal, and therapy was frequently prolonged beyond recommendations in all scenarios except for castration.
Judicious use of antimicrobials through appropriate routes of administration
Fig 4. Using antimicrobials as effectively as possible helps to reduce their use overall. For septic arthritis, intravenous regional perfusion of antimicrobials can achieve very high concentrations within a specific limb. This involves placing a temporary tourniquet to reduce blood flow away from the area while the antimicrobial is injected into a nearby blood vessel. The technique is suitable for some but not all antimicrobial drugs.
Due to increasing isolation of MDR organisms, research into local therapy of “reserved” classes of antimicrobials is of interest. Intravenous regional limb perfusion of ceftiofur sodium may be appropriate for septic arthritis but is less clear cut for osteomyelitis.
Oral and rectal administration of antimicrobials are common means to provide cost-effective and convenient treatment options for owners. However, these routes of administration can lead to variable absorption and therefore have the potential for subtherapeutic concentrations. Rectal administration of some antimicrobials has been explored in order to provide antimicrobials to horses with diseases that prevent oral administration, such as small intestinal problems or to provide an alternative for horses that find drugs unpalatable and go off their feed. Metronidazole is one of the few drugs for which pharmacokinetic data following rectal administration have been published, but the optimal dosing regimens via this route have yet to be determined.
Clinical conclusions
Given the increasing prevalence of resistant bacteria affecting the equine population, judicious use of antimicrobials is necessary. Trainers and vets must work together to implement this, otherwise before long, we will find we have no effective drugs left. Firstly, in any given situation, we should question whether antibiotics are really necessary.
Appropriate antibiotic selection, as well as choosing the correct dose, frequency, duration and route of administration should all be considered. Veterinarians should encourage culture and sensitivity testing to allow for guided and narrow spectrum therapies whenever possible. It is also important to keep up-to-date with the latest information on drug treatment schedules and be prepared to modify and adapt as new information becomes available. Appropriate antimicrobial stewardship in veterinary medicine will ensure the availability and legal use of antimicrobials remains an option for our equine patients.
Experiences with a new surgical technique for ‘Wobblers’ horses
By Lynn Pezzanite
Wobbler syndrome, also known as cervical vertebral compressive myelopathy (CVCM), is the most common cause of neurological disease in horses and affects many breeds. Although numerous spinal surgeries are performed on humans, this is the only condition of the spinal cord for which surgery in horses is often performed.
Wobbler syndrome involves compression of the spinal cord due to narrowing or abnormal development of the spine in the neck, which results in neurologic deficits—specifically ataxia. Ataxia is a term used by veterinarians to describe incoordination and inability of an animal to properly place their legs and maintain balance when they are standing and walking. It is easy, therefore, to see why horsemen describe CVCM horses as “wobblers.” CVCM has been described in many breeds, and it was estimated to affect up to 3% of thoroughbreds in one UK study. There is a high prevalence in young male horses, and these horses comprise 75 to 80% of cases. The condition negatively affects athletic performance, and up to 2/3 of horses diagnosed with CVCM are euthanised due to severity of the ataxia or perceived poor response to therapy and subsequent loss of use of the horse. Treatment recommendations are controversial due to the fear that horses cannot recover function when diagnosed with this condition, as well as concerns regarding the cost of treatment, its invasiveness and complications associated with current surgical procedures. Also, at the current time, it is still very unlikely a veterinarian can accurately predict the degree of improvement and prognosis for a specific horse undergoing treatment. Furthermore, veterinarians do not always agree amongst themselves how severe the ataxia is, which makes it even more difficult to measure improvement following treatment and compare treatments. Despite these concerns, there are many horses that do improve and return to athletic use after neck spinal surgery.
What are the current options for spinal surgery?
The goal of spinal surgery for CVCM is to remove the ability of two vertebral bodies to move by fusing the two adjacent bones together. The result is that over time, the two bones and joints will change in configuration, the fused bones shrink and more space becomes available for the spinal cord. By removing the compression of the spinal cord, neurological function improves. Current surgical treatments for CVCM include methods for ventral interbody fusion: kerf cut cylinders and ventrally placed locking compression plate and dorsal laminectomy (the top portion of the vertebral body is removed entirely to reduce any compression on the spinal cord). Fusion with using the kerf cut cylinder remains the most commonly performed surgical procedure for cervical stabilisation, but this does not provide stability when the spine is in extension. Locking compression plate technologies are difficult to apply due to the shape of the vertebral body and limited flexibility in placement of the fusion construct and the associated screws. Despite great advancements in equine surgery over the past years, these surgical methods for equine cervical stabilisation require specialised equipment and extensive surgeon experience and still have a high risk of complications, including implant migration or failure and vertebral fracture with a high chance of associated horse fatality.
The goal of spinal surgery for CVCM is to remove the ability of two vertebral bodies to move by fusing the two adjacent bones together
Recent developments in spinal surgery
Because CVCM is relatively common and there is huge interest in returning affected horses to athletic function, there is a demand to develop surgical techniques that are less technically challenging while reducing complications associated with surgery to safely return horses affected by CVCM to their intended use. Overall, there remains room for improvement in surgical treatment of CVCM to both increase biomechanical stability and reduce complications associated with implant placement.
A new technique for spinal surgery
In a recent pilot study by our group at the PreClinical Surgical Research Laboratory at Colorado State University (Fort Collins, CO, USA), a new technique using advanced surgical implants known as pedicle screws and connecting rods with an interbody fusion device (IFD) were evaluated as an alternative to current techniques for cervical fusion in horses. The idea to use these novel implants came from human surgery, where interbody fusion devices are considered the standard technique for lumbar spine fusion in people, resulting in improved success rates in neurologic function and return to activity. The IFD device was evaluated initially in four horses, showing that the construct integrated with surrounding bone within eight months and did not result in any severe complications, such as implant failure, migration or fracture (as has been reported with other techniques). In addition, we noted that the polyaxial pedicle screw head allowed for increased screw placement options compared to previously described techniques. In particular, this is an improvement compared to the locking compression plate technology, which is limited by the conformation of the ventral keel of the cervical vertebrae. The results obtained in this pilot study prompted further investigation of polyaxial pedicle screw and rod technology in equine patients clinically affected by CVCM.
The Colorado team’s results
We found 10 horses at the Colorado State University Veterinary Teaching Hospital that were diagnosed with Wobbler syndrome based on examination and diagnostic imaging including x-rays, myelogram, and CT scan. The owners of the horses approved to have them undergo this new surgery with placement of the IFD and polyaxial pedicle screw and rod construct. The 10 horses were closely followed, and clinical outcomes and owner reports were recorded and described in our recent publication in Equine Veterinary Journal.
The breeds of horses treated included warmbloods, Tennessee Walkers, Arabians and quarter horses. No horses in this case population were intended as racehorses. The median age of horses at the time of surgery was two years (24 months, range 12-168). Male horses were overrepresented as is typical for CVCM, with four geldings, four stallions and two mares treated. Preoperative grade of ataxia ranged from 1 to 3 out of 5 based on the Modified Mayhew neurological grading scale. Surgical fusion was performed at one site in three horses and two sites in seven horses. In 6 out of 8 horses with ≥1-year follow-up, ataxia improved by 1–3 grades, with an average improvement of 1.25 grades. In four horses, ataxia improved to grade 0 (normal) or 1 (mild ataxia). In two horses, the gait was unaffected, but neck comfort improved according to owner follow-up. There were no fatal complications associated with the placement of implants. Complications encountered included swelling around the incision site (seroma), pain and fever. Although we found more serious complications including screw breakage in two horses, a vertebral fracture in one horse, and implant infection in one horse, none of these horses required additional surgical procedures to remove the implants. Two horses were euthanised within the first year after surgery. In one horse with severe neurological deficits preoperatively, surgery did not result in improvement of signs; and the horse was euthanised at six weeks postoperatively. The second horse developed upper respiratory tract obstruction immediately following general anesthesia and was euthanised at the time.
Long-term follow-up with owners was performed by phone and survey consultation. All eight owners for which at least one year follow-up after surgery was available, reported that their horse’s clinical signs and quality of life were improved, and for all horses the level of exercise was increased since surgery. Five horses were being ridden at the time of follow-up, and one additional juvenile horse was beginning training. All four horses that had been ridden before surgery had improved under saddle. Overall, owner satisfaction with the procedure was reported as excellent in five cases or good in two cases, with one owner not responding to the question. All eight owners reported that they were overall positive about the procedure and would recommend this surgery to other horse owners in the future.
This new surgical technique to treat horses with Wobbler syndrome resulted in at least one grade of gait improvement in 6/10 cases and 6/8 cases for which ≥1-year follow-up was available, which is a similar result when compared to other methods. Advantages of this surgical procedure over others to treat this syndrome in horses include that this technique requires less bone removal from the vertebral column and that the implant itself (polyaxial screw head) may be more easily applied to the vertebral body, as its shape can be varied and so can be tailored to each individual horse. Importantly, this technique offers greater stability in two planes (tension and compression), which is not provided by other techniques such as the Bagby basket or kerf cut cylinder. There were no fatal complications related to implant placement in this procedure. This is in contrast to other techniques such as the basket or kerf cut cylinder, where euthanasia of the horse is the more typical outcome if the implant fails and vertebral fracture occurs due to the extent of damage that usually results in spinal cord injury with subsequent severe neurologic signs. In summary, this technique may represent a safer alternative to current techniques of ventral interbody fusion while achieving similar outcomes in performance. Polyaxial pedicle screw and rod systems for cervical fusion should be considered as an alternative to minimise fatal complications associated with surgery while achieving one to three grades of improvement in neurological signs in horses with Wobbler syndrome. However, this study was performed in a small number of horses, so continued study of this method remains critical, as well as further development and optimisation of other surgical techniques that may result in lower frequency of complications and greater neurologic improvement.
Pezzanite, et al, Outcomes after cervical vertebral interbody fusion using an interbody fusion device and polyaxial pedicle screw and rod construct in 10 horses (2015-2019) https://beva.onlinelibrary.wiley.com/doi/10.1111/evj.13449
Orthopaedic problems in young Thoroughbreds
Helping these future athletes achieve a protective conformation is vital with respect to their welfare, athletic career and sales potential: Orthopaedic conditions have the potential to blight a promising athletic career and prevent young horses reach their full potential. Early diagnosis and management are critical if horses are to be given the best chances of a successful and long career. And this, of course, depends on horsemen being able to pick up on problems as early as possible so they can be dealt with effectively. The Beaufort Cottage Educational Trust is a charity that aims to help disseminate knowledge in the Thoroughbred breeding and racing communities with the ultimate goal of improving horse welfare.
Each year, the charity organizes the Gerald Leigh Memorial lectures which are fantastic resources for horsemen. The lecture series is supported by the Gerald Leigh Trust in honor of Mr. Leigh's passion for the Thoroughbred horse and its health and welfare. Most years, the lectures are presented in person in an event at the UK’s National Horseracing Museum in Newmarket; but for 2021, an in-person gathering was not possible and instead, the lectures are available online. For 2021, the charity chose the theme of orthopaedic problems, which are such a common challenge in young Thoroughbreds.
Angular Limb Deformities: Evaluation and treatment in foals and yearlings
Recognizing, diagnosing and understanding angular limb deviations in young Thoroughbreds are critical skills for horsemen and an important part of both stud management and veterinary care. Angular limb deformities (ALD) refer to deviation of the limb in its frontal plane, or side to side when evaluating the individual from the front or back. A varus deformity is a medial deviation of the limb below the location of the problem (e.g., toeing in), whereas a valgus deformity is a lateral deviation of the limb below the location of the deformity (e.g., toeing out). Angular limb deformities must be distinguished from a flexural limb deformity, which is in the sagittal plane, i.e., from front to back when evaluating the individual from the side.
How do ALD occur?
ALD can be both congenital and acquired. Congenital means the condition has been present from birth and causes include incomplete ossification or immaturity of the small cuboidal bones, which make up the hocks and knees as well as weakness of the ligaments supporting the joints and periarticular laxity. These issues tend to result in valgus knees and hocks. We also know that ALD can be inherited and that as a breed, Thoroughbreds tend to be varus (toe in).
Acquired ALD develop after birth and come about through overloading of the physis (growth plate), which is usually caused either from hard ground, an over-conditioned foal or a combination of the two. The biomechanics of equine limb lead horses to bear more weight through the inside of the leg; therefore, the inside of the growth plate, which is inhibited more than the outside and when there is overloading the net effect is that the foal will toe in.
How do ALD impact a foal’s future career?
Carpal and fetlock injuries in racing Thoroughbreds account for a large majority of the reasons racehorses spend time out of training. Intervening while foals are growing and developing to help them achieve a protective conformation gives them the best chance of maximizing their potential and enjoying their racing career.
Diagnosis of ALD
Evaluating young stock is certainly best achieved using a team approach involving owners/managers, farriers and veterinarians. Regular evaluation from a young age is key, as is examination of the foal while static and while walking. Severe deviations should also be evaluated radiographically.
Treatment of ALD
Conservative treatment options can include exercise restriction, corrective farriery and nutritional management. Hoof correction and toe extensions can be extremely helpful in managing foals and yearlings with minor deviations; and farriery can often correct such issues without needing to resort to surgical treatment options.
The surgical treatment of choice for correcting ALD is the transphyseal screw. In general, it achieves the most effective and cosmetic outcome of the surgical options. The procedure involves placing a screw across the growth plate on the side of the leg that is growing too fast. For example, for a foal that is toeing in, the screw is placed on the outside of the leg. This allows the inside of the growth plate to grow faster and so correct the deviation. The screws are placed under a short general anesthetic. The screw does need to be removed to avoid over-correction, but often they can be removed with the horse standing using a mild sedative once the desired correction is achieved.
Osteochondrosis – recent advances and diagnosis
Osteochondrosis is one of the most important developmental diseases in young athletic horses. It occurs in young, large-breed horses, including Thoroughbreds, and can cause a variety of clinical signs. The age at which the disease starts to cause clinical signs varies from a young foal to horses over 10 years old. This is because lesions can remain silent and only cause clinical signs later on in life. But even in the absence of any clinical signs, the pathological lesions will have been present since the horses reached skeletal maturity.
How does osteochondrosis affect athletes?
Osteochondrosis often starts to cause problems when the horse is put into training—when they are athletically challenged. This age will differ for different populations, starting earlier in Thoroughbred racehorses than in Warmbloods destined for sports horse disciplines. Often the horse will be sound, or can experience different degrees of lameness and may present with joint effusion. This disease affects more than one joint in an individual in over 50% of cases, and it usually occurs in the same joint on the contralateral limb; but it can also affect multiple different joints.
How does osteochondrosis develop?
In foals, areas of growth cartilage within the joints will continue to ossify (become bone) after birth. When this process is complete and the animal is skeletally mature, a thin layer of normal articular cartilage will remain supported by subchondral bone. Osteochondrosis is caused by a “failure of endochondral ossification,” which simply means the growth cartilage fails to become healthy bone. A defect, with or without a fragment, is then created in the articular surface of the bone. This dynamically changing area is susceptible to trauma or high biomechanical loads. Recent advances in research, carried out in Norway by Dr. Olstad, suggest that failure of endochondral ossification is likely caused by loss of blood supply to these areas of growth cartilage, which prevents it from ossifying. This has been linked to a heritable predisposition, among other factors such as rapid growth, dietary imbalance, exercise, environment and prior joint sepsis.
Diagnosis of osteochondrosis
Thorough clinical examination and radiography remain at the forefront of osteochondrosis diagnosis. This disease occurs at joint-specific predilection sites as a result of site-specific biomechanical forces and differences in the age at which that site becomes skeletally mature. For example, in the femoropatellar joint (pictured), the most common site of osteochondrosis is the lateral trochlear ridge of the femur. This is predilected by the thick cartilage surface, later age of maturation/ossification, and by the shear forces the patella exerts on the ridge as the stifle flexes and extends. Ultrasonography can also be very sensitive in detecting osteochondrosis in the stifle. Research performed by Dr. Martel in Canada suggests early detection of subclinical lesions in the stifle have been found in foals aged 27-166 days old.
Management of osteochondrosis
Lesions can spontaneously resolve, and the majority will have done so by 12 months old. Otherwise, management recommendations to limit lesion development include keeping horses exclusively at pasture up to 1 year old, not using rough terrain, in large group sizes (>3 brood mares) or in a large pasture size (large pasture size > 1 hectare before 2 weeks old and > 6 hectare before 2 months old). Strict box rest is discouraged, and a convalescence paddock of 33ft x 56ft (10m x 17m) for 60-90 days may help stabilize lesions.
Conclusion
Gerald Leigh was an incredibly successful Thoroughbred breeder and owner based in the UK. The 2021 lectures honoring his passion for the Thoroughbred provide a useful update for horsemen on two common conditions of the young Thoroughbred and add to the contribution the charitable trust established by Mr. Leigh’s family, which continues to make in supporting the Thoroughbred industry.
CLICK HERE to return to issue contents for this issue
Can we use biomarkers to predict catastrophic racing injuries?
By Holly Wiemers
University of Kentucky study shows association between mRNA biomarkers and catastrophic injuries in Thoroughbred racehorses— a positive step forward in the development of a pre-race screening tool.
Catastrophic injuries in Thoroughbred racehorses is a top-of-mind concern for the global racing industry and its fans. That sentiment is shared by researchers at the University of Kentucky and their collaborators, who are working to learn more about changes happening at a cellular level that might indicate an injury is lurking before it becomes career or life ending. Could it be possible to identify an early marker or signal in horses at risk of catastrophic injury, allowing for intervention before those injuries happen? And, if so, might this type of detection system be one that could be implemented cost effectively on a large scale?
According to Allen Page, DVM, PhD, staff scientist and veterinarian at UK’s Gluck Equine Research Center, the short answer to both questions is that it looks promising.
Allen Page
To date, attempts to identify useful biomarkers for early injury detection have been largely unsuccessful. However, the use of a different biomarker technology, which quantifies messenger RNA (mRNA), was able to identify 76% of horses at risk for a catastrophic injury. An abstract of this research was recently presented at the American Association of Equine Practitioners’ annual meeting in December 2020 and the full study published January 12 in the Equine Veterinary Journal (www.beva.onlinelibrary.wiley.com/journal/20423306).
In this initial research—which looked at 21 different mRNA markers selected for their roles in encoding proteins associated with inflammation, bone repair and remodeling, tissue repair and general response to injury— three markers showed a large difference in mRNA levels between injured and non-injured horses.
For almost four years, Page and his University of Kentucky colleagues have been analyzing blood samples from almost 700 Thoroughbred racehorses. These samples, collected by participating racing jurisdictions from across the United States, have come from both catastrophically injured and non-injured horses in a quest to better understand changes that might be happening at the mRNA level and if there are any red flags which consistently differentiate horses that suffer a catastrophic injury. …
CLICK HERE to return to issue contents or sign up below to read this article in full
BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 60 (PRINT)
$6.95
ISSUE (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - ONLY $24.95
Roarers - surgery for recurrent laryngeal neuropathy -impact and outcomes
By Safia Barakzai
Recurrent laryngeal neuropathy (RLN), more commonly known as “roaring”, “laryngeal paralysis” and “laryngeal hemiplegia” is a disorder affecting primarily the left recurrent laryngeal nerve in horses >15hh. This nerve supplies the muscles that open and close the left side of the larynx. The right recurrent laryngeal nerve is also now proven to be affected, but only very mildly, thus affected horses very rarely show signs of right-sided dysfunction. Horses with RLN become unable to fully open (abduct) the left side of their larynx. During exercise they then make abnormal inspiratory noise due to collapse of both the vocal fold(s) and the left arytenoid cartilage (Fig. 1), and airflow to the lungs can become severely obstructed in advanced cases. There is a proven genetic component to RLN, but in many cases the disease progresses over months or years. The age at which clinical signs become apparent is highly variable. Foals can show endoscopic and pathologic evidence of RLN, but some horses do not develop clinical disease until >10 years old. Severity of disease can be reasonably estimated using endoscopy in the resting horse (grades 1-4), but the gold standard for assessing this disease is endoscopy during exercise, when the high negative pressure—generated when breathing—test the affected laryngeal muscle, which is trying its best to resist the “suctio”’ effect of inspiration (Fig. 1).
Horse undergoing exercising endoscopy to ascertain how the left arytenoid performs when the airway is under pressure. Inset photos show resting (top) and then exercising endoscopy (bottom) of a larynx with grade D arytenoid collapse (green arrow) with additional deformation of the arytenoid cartilage shape and bilateral vocal fold collapse (red arrows).
During exercise, RLN is graded from A to D, depending on how much the left side of the larynx can open (Table 1).
• Treatment of RLN
TABLE 1: Grades A-D of laryngeal abduction during exercise. Figures c/o F. Rossignol.
Traditionally, left-sided ventriculocordectomy (“Hobday”/ ventriculectomy plus vocal-cordectomy surgery) and laryngoplasty (“tie-back”) surgeries have been used to treat the disorder, depending on which structures are collapsing and how severely. The intended use of the horse, the budget available and other concerns of the owner/trainer also come into play. New techniques of providing a new nerve supply (“re-innervating”) to the affected muscle are now being trialled in clinical cases. Pacing the muscle with an implanted electronic device has also been attempted in research cases.
• Ventriculocordectomy
Ventriculocordectomy is commonly now referred to as a “Hobday” operation; however, the “Hobday” actually only refers to removal of the blind ending sac that constitutes the laryngeal ventricle. Currently, surgeons tend to remove the vocal cord as well as the ventricle, because it is vocal cord collapse that creates the “whistling” noise. It is a relatively straightforward surgery to perform with minimal risks and complications for the patient. In the last 15 years, there has been a shift to performing it in a minimally invasive way, using a diode laser under endoscopic guidance in the standing sedated horse rather than with the conventional method, via an open laryngotomy incision on the underside of the neck with the horse under a general anesthetic. However, transendoscopic laser surgery is technically difficult with a very steep learning curve for the surgeon. All ventriculocordectomies are not equal (Fig. 2) and for both laser and ‘open surgery’ methods, incomplete resection of the fold can leave behind enough tissue to cause ongoing respiratory noise and/or airway obstruction after surgery.
Two horses after ventriculocordectomy surgery. The horse on the left has an excellent left-sided ventriculocordectomy, with complete excision of the vocal fold tissue (black arrow). The right cord is intact, but the right ventricle has been removed (‘Hobday’). The horse on the right has bilaterally incomplete vocalcordectomies, with much of the vocal fold tissue left behind (green arrows).
Sports horses, hunters and other non-racehorses were often previously recommended to have a ventriculocordectomy performed rather than a laryngoplasty, even if they had severe RLN. This decision was often made on the grounds of cost, but also due to fear of complications associated with laryngoplasty (‘tie-back’ surgery). A new study has shown that for horses with severe RLN, a unilateral ventriculocordectomy is actually extremely unlikely to eliminate abnormal noise in severely affected horses, because the left arytenoid cartilage continues to collapse.3 The authors recommended that laryngoplasty plus ventriculocordectomy is a better option than ventriculocordectomy alone for all grade C and D horses if resolution of abnormal respiratory noise and significant improvement of the cross sectional area of the larynx are the aims of surgery.3
Advancements in laryngoplasty (‘tie-back’) surgery
Laryngoplasty is indeed one of the most difficult procedures that equine surgeons perform, and suffice to say that with such an advanced surgery, using a registered specialist veterinary surgeon that has considerable experience in airway surgery will likely minimise the chances of a negative outcome. Laryngoplasty surgery has an unjustified poor reputation in my opinion, …
CLICK HERE to return to issue contents or sign up below to read this article in full
BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 60 (PRINT)
$6.95
ISSUE (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - ONLY $24.95
The differences between a healthy / unhealthy biome. We learn how gene sequencing technology can reveal common gastrointestinal disease.
By Carol Hughes PhD
Gastrointestinal diseases and upsets are common in Thoroughbred racehorses, causing discomfort, loss of performance and even mortality. Every common gastrointestinal disease can be linked back to disturbances (dysbiosis) of the gut bacteria. Currently, new gene technology is driving research at an intense rate, providing new insights into the equine microbial community (1) and providing both trainer and the vet with a powerful and accurate analytical tool to improve health and manage disease. The gastrointestinal tract of the horse is colonized by trillions of microorganisms, which includes 1,000-1,500 different species, making up around 95% of the biome; the other 5% are made up of archaea, protozoa, fungi and viruses. Though most studies concentrate on identifying species of bacteria and linking to health and disease. Other members of the biome have equally important roles to play. In the racehorse, a major player is the Enterobacteria phage PhiX174, which is a bacterial virus that protects the horse against E-coli.(2) The microbial community has co-evolved with the host, performing essential and vital activities such as the extraction of energy and nutrients from foodstuff, synthesis of vitamins, interaction with the immune system and cross talk with the brain, which is thought to affect temperament and behavior. Taxonomic and functional compositions of the gut microbiome are rapidly becoming viable indicators of horse health and disease. Each member of the microbial community has a different but synergistic role, which is beneficial to the health of the horse; e.g., the fungi break down the indigestible parts of forage plants, such as the polysaccharides, while the ciliate protozoa contribute to the process by producing a wide range of enzymes that the horse is unable to make, impacting and benefitting the immune system. Microbial fermentation of cellulose, hemicellulose and lignin reduces the structural and non-structural plant wall material into carbohydrates, proteins (amino acids) and lipids, and produces volatile and short chain fatty acids,(2a) which are the primary source of energy for the horse. The bacteria contribute the most to the degradation of ingested food, producing the final components of the fermentation process, which are acetic, propionic and butyric acid, methane and carbon dioxide. The gastrointestinal tract of the horse is sensitive to change, stress, environment and medication, which cause imbalances or dysbiosis.(3)
Fig 1: Image of the analysis of the microbiome of a Grade 1 horse, compared to a non-group horse.
Establishing or profiling a healthy baseline in the horse is difficult as variations exist between individuals, breeds, diets and locations; the Thoroughbred racehorse is a very different animal to the Shetland pony or an Irish Draught. Fitness training alters the microbiome further; for these reasons it is important to study the Thoroughbred as a population separate from other breeds and to analyze, where possible, racehorses training in a similar environment and location. With this in mind, since 2017 there has been an ongoing project to study and profile the microbial populations of over 1,000 racehorses based in Newmarket, throughout the racing season; and the data produced has been used to develop profiles of the differences between a healthy/unhealthy biome. The project utilizes the cutting-edge Illumina MiSeq technology, which is the most accurate and up-to-date, preferred by genomic researchers around the world.
THE BIOME IN HEALTH ELITE RACEHORSES HAVE HIGHER LEVELS OF A SUPER-PHYLUM BACTERIA
Questions asked...
Elite racehorses are trained to achieve peak fitness, but is it possible that they can gain an extra edge from the input of the hind gut bacteria?
How different is the microbiome of a Grade 1 horse, and is it possible to identify the bacteria responsible for the extra edge?
Answers found...
Human scientists have known for some time that the microbiome of an elite human athlete is different,(4) with faster metabolic pathways (amino acids and carbohydrates) and higher levels of fecal metabolites (microbial-produced short-chain fatty acids) acetate, propionate and butyrate associated with enhanced muscle fitness. The human and elite equine athlete do share similar microbial profiles, having higher percentages of the bacteria that manufacture short-chain fatty acids and higher levels of the super-phylum verrucomicrobia; these increase as the season/training progresses. What is known about this super-phylum? It has two main members: Methylacidiphilaceae and Akkermansia
1) Verrucomicrobia Methylacidiphilaceae thrive and proliferate on the ammonia produced from the degradation of starch and protein,(5) whereas starch produces very high levels of ammonia. The bacteria make enzymes (ammonia monooxygenase),(6) which convert ammonia into nitric oxide.(7) The nitric oxide has three major benefits to a racehorse:
a. Helps repair and renew the gut wall (8)
b. Enhances performance and increases exercise tolerance (9)
c. Improves vascular function and metabolism (10)
2) Verrucomicrobia Akkermansia is a mucus-eating specialist, living and thriving within the gut wall, digesting mucin from the mucosal lining (10a) with a unique ability to metabolise galactose and melibiose (11) for energy. Akkermansia in the human biome significantly increases the numbers of metabolic pathways. Horses with gastric ulcers have very low levels, perhaps indicating its function in both performance and disease. …
CLICK HERE to return to issue contents or sign up below to read this article in full
ISSUE 59 (PRINT)
$6.95
ISSUE 59 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - ONLY $24.95
Outlook for stem cell therapy - its role in tendon regeneration - different treatments for horse tendon injuries
By Dr Debbie Guest
Tendon injuries occur very commonly in racing thoroughbreds and account for 46% of all limb injuries. The superficial digital flexor tendon (SDFT) is the most at risk of injury due to the large strains that are placed upon it at the gallop. Studies have reported that the SDFT experiences strains of up to 11-16% in a galloping a thoroughbred, which is very close to the 12-21% strain that causes the SDFT to completely rupture in a laboratory setting.
An acute tendon injury leads to rupture of the collagen fibres and total disruption of the well organised tendon tissue (Figure 1). There are three phases to tendon healing: an inflammatory phase that lasts for around one week, where new blood vessels bring in large numbers of inflammatory blood cells to the damaged site—a proliferative phase that lasts for a few weeks, where the tendon cells rapidly multiply and start making new collagen to replace the damaged tissue; and a remodelling phase that can last for many months, where the new collagen fibres are arranged into the correct alignment and the newly made structural components are re-organised.
Figure 1. A) The healthy tendon consists predominantly of collagen fibres (light pink), which are uniformly arranged with tendon cells (blue) evenly interspersed and relatively few blood vessels (arrows). B) After an injury the collagen fibres rupture, the tissue becomes much more vascular, promoting the arrival of inflammatory blood cells. The tendon cells themselves also multiply to start the process of rebuilding the damaged structure.
After a tendon injury occurs, horses need time off work with a period of box rest. Controlled exercise is then introduced, which is built up slowly to allow a very gradual return to work. This controlled exercise is an important element of the rehabilitation process, as evidence suggests that exposing the tendon to small amounts of strain has positive effects on the remodelling phase of tendon healing. However, depending on the severity of the initial injury, it can take up to a year before a horse can return to racing. Furthermore, when tendon injuries heal, they repair by forming scar tissue instead of regenerating the normal tendon tissue. Scar tissue does not have the same strength and elasticity as the original tendon tissue, and this makes the tendon susceptible to re-injury when the horse returns to work. The rate of re-injury depends on the extent of the initial injury and the competition level that the horse returns to, but re-injury rates of up to 67% have been reported in racing thoroughbreds. The long periods of rest and the high chance of re-injury therefore combine to make tendon injuries the most common veterinary reason for retirement in racehorses. New treatments for tendon injuries aim to reduce scar tissue formation and increase healthy tissue regeneration, thereby lowering the risk of horses having a re-injury and improving their chance of successfully returning to racing.
Over the past 15 years, the use of stem cells to improve tendon regeneration has been investigated. Stem cells are cells which have the remarkable ability to replicate themselves and turn into other cell types. Stem cells exist from the early stages of development all the way through to adulthood. In some tissues (e.g., skin), where cells are lost during regular turnover, stem cells have crucial roles in normal tissue maintenance. However, in most adult tissues, including the tendon, adult stem cells and the tendon cells themselves are not able to fully regenerate the tissue in response to an injury. In contrast, experimental studies have shown that injuries to fetal tissues including the tendon, are capable of undergoing total regeneration in the absence of any scarring. At the Animal Health Trust in Newmarket, we have an ongoing research project to identify the differences between adult and fetal tendon cells and this is beginning to shed light on why adult cells lead to tendon repair through scarring, but fetal cells can produce tendon regeneration. Understanding the processes involved in fetal tendon regeneration and adult tendon repair might enable new cell based and/or therapeutic treatments to be developed to improve tendon regeneration in adult horses.
In many tissues, including fat and bone marrow, there is a population of stem cells known as mesenchymal stem cells (MSCs). These cells can turn into cells such as bone, cartilage and tendon in the laboratory, suggesting that they might improve tendon tissue regeneration after an injury. MSC-based therapies are now widely available for the treatment of horse tendon injuries. However, research has demonstrated that after injection into the injured tendon, MSCs do not turn into tendon cells. Instead, MSCs produce factors to reduce inflammation and encourage better repair by the tissue’s own cells. So rather than being the builders of new tendon tissue, MSCs act as the foreman to direct tissue repair by other cell types. Although there is some positive data to support the clinical application of MSCs to treat tendon injuries in horses, placebo controlled clinical trial data is lacking. Currently, every horse is treated with its own MSCs. This involves taking a tissue biopsy (most often bone marrow or adipose tissue), growing the cells for 2-4 weeks in the laboratory and then injecting them into the site of injury. This means the horse must undergo an extra clinical procedure. There is inherent variation in the product, and the cells cannot be injected immediately after an injury when they may be the most beneficial.
To allow the prompt treatment of a tendon injury and to improve the ability to standardise the product, allogeneic cells must be used. This means isolating the cells from donor horses and using them to treat unrelated horses. Experimental and clinical studies in horses, mice and humans suggest that this is safe to do with MSCs, and recently an allogeneic MSC product was approved for use in the EU for the treatment of joint inflammation in horses. These cells are isolated from the circulating blood of disease-screened donor horses and are partially turned into cartilage cells in the laboratory. They are then available “off the shelf” to treat unrelated animals. Allogeneic MSC products for tendon injuries are not yet available, but this would provide a significant step forward as it would allow horses to be treated immediately following an injury. However, MSCs exhibit poor survival and retention in the injured tendon and improvements to their persistence in the injury site, and with a better understanding of how they aid tissue regeneration, they are required to enable better optimised therapies in the future.
Our research has previously derived stem cells from very early horse embryos (termed embryonic stem cells, ESCs. Figure 2). ESCs can grow in the laboratory indefinitely and turn into any cell type of the body. These properties make them exciting candidates to provide unlimited numbers of cells to treat a wide range of tissue injuries and diseases. Our experimental work in horses has shown that, in contrast to MSCs, ESCs demonstrate high survival rates in the injured tendon and successfully turn into tendon cells. This suggests that ESCs can directly contribute to tissue regeneration.
Figure 2. A) A day 7 horse embryo used for the isolation of ESCs. Embryos at this stage of development have reached the mare’s uterus and can be flushed out non-invasively. B) “Colonies” of ESCs can grow forever in the laboratory.
To understand if ESCs can be used to aid tendon regeneration, they must be shown to be both safe and effective. In a clinical setting, ESC-derived tendon cells would be implanted into horses that were unrelated to the original horse embryo from which the ESCs were derived. The recipient horse may therefore recognise the cells as “foreign” and raise an immune response against them. Using laboratory models, we have shown that ESCs which have been turned into tendon cells do not appear recognisable by the immune cells of unrelated horses. This may be due to the very early developmental stage that ESCs originate from, and it suggests that they would be safe to transplant into unrelated horses.
To determine if ESCs would be effective and improve tendon regeneration, without the use of experimental animals, we have established a laboratory system to make “artificial” 3D tendons (Figure 3).
Figure 3. Artificial 3D tendons grown in the laboratory are used to study different sources of tendon cells and help us work out how safe and effective an ESC-based therapy will be. A) Artificial 3D tendons are 1.5 cm in length. B) a highly magnified view of a section through an artificial tendon showing well-organised collagen fibres in green and tendon cells in blue.
ESC-tendon cells can produce artificial 3D tendons just as efficiently as adult and fetal cells, and this system allows us to make detailed comparisons between the different cell types. …
CLICK HERE to return to issue contents
ISSUE 58 (PRINT)
$6.95
ISSUE 58 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - ONLY $24.95
Don’t test your luck - equine stomach ulcer diagnosis deserves a proven treatment
In association with
Ulceration of the stomach is a common health problem among athletic horses around the world. The reported prevalence of ulcers in racing Thoroughbred or Standardbred horses is high, commonly quoted between 55% and 90%.1 The economic impact of this disease is difficult to calculate because the impact on athletic performance has not been accurately determined. However, there are well-defined costs attributable to diagnosis, medication and the labor required for treatment.
When it comes to cost associated with ulcer medication, it is often said that “the most expensive treatments are the ones that don’t work.” Proton pump inhibitors, of which omeprazole is considered the best studied in horses, make up one of the most commonly used classes of drugs for equine gastric ulcers. But while there are several omeprazole products on the market, are they all created equal? The simple answer is no.
Animal drugs are approved by the U.S. Food and Drug Administration (FDA) for use in specific species to treat particular conditions. To be legally marketed, animal drugs, with few exceptions, must meet the FDA’s stringent safety and efficacy standards. However, many animal drugs are manufactured and sold by compounding pharmacies, and as such are not FDA-approved brand-name drugs.
Compounded drugs can serve an important medical need for patients in certain circumstances, but they do not have the same safety, quality and effectiveness assurances as FDA-approved drugs. In addition, the FDA does not review the manufacturing methods used to make them or the accuracy of their labeling. Therefore, poor compounding practices can result in serious drug quality problems — such as bacterial or fungal contamination, or drugs that have either too much or too little active ingredient(s) — which can lead to serious patient injury and death. The American Association of Equine Practitioners (AAEP) advises: “Limit the use of compounded drugs to unique needs in specific patients.”2
There have been various headlines, articles, and research studies over the years regarding improperly compounded medications and their associated risks to horse health and welfare, and their financial implications. When it comes to stability and efficacy, compounded omeprazole products often don’t measure up. Compounded and illegally manufactured omeprazole products often have safety, efficacy, and production quality control problems like incomplete filling of syringes, air pockets, and variations in homogeneity (see Figure 1.).3
While it is vital to take every step to protect your horse's gastric health, it is a good idea to look closely when considering compounded omeprazole products. In laboratory testing, some compounded omeprazole products contained as little as 6% of their labeled value (see Figure 2.).4
In a 60-day in-vitro study comparing five compounded pastes to Gastrogard® (omeprazole), compounded formulations started as low as 63% of labeled concentration on day 0 and fell to as low as 17% on day 60.5 GASTROGARD concentrations remained stable and at label concentration throughout the study.
Figure 2. Active omeprazole vs. label claim in 10 compounded products
In another study involving 32 adult racehorses in active training, results suggested that while administration of Gastrogard® (omeprazole) was effective in promoting healing of gastric ulcers in these horses, administration of the compounded omeprazole suspension was ineffective.6
GASTROGARD is one of the most widely used prescription medications and the only FDA-approved oral formulation of omeprazole available in the United States for horses.
The omeprazole in GASTROGARD is specially protected allowing it to reach the site of absorption in the small intestine so that it can suppress gastric acid production to a level that allows ulcers to heal.7
In addition to premarket review, FDA-approved animal drugs are subject to requirements once they are on the market. For instance, manufacturers must submit adverse event reports — including reports of product defects — and provide information to the FDA related to safety, effectiveness, and manufacturing quality throughout the product’s lifetime. These reports allow the FDA to continue to monitor the safety and effectiveness of the drug after approval. Unlike manufacturers of FDA-approved animal drugs, compounders are not required to report adverse events and product defects.
What are the advantages of FDA-approved drugs?8
• Safety and efficacy based on thorough scientific review
• Meet quality, purity and potency specifications
• Continual monitoring ensures product performance
• Consistent manufacturing standards
Furthermore, FDA believes it is critically important to disclose risk information of prescription drugs appropriately and effectively to healthcare professionals and consumers. Important safety information of the drug can be found in its entirety on the package label and/or package insert. GASTROGARD is labeled for use in horses and foals 4 weeks of age and older. The safety of GASTROGARD paste has not been determined in pregnant or lactating mares. GASTROGARD is not to be used in horses intended for human consumption.
Knowledge is power when it comes to deciding whether to use compounded versus FDA-approved drugs. Do not hesitate to ask your veterinarian about the medications being used in your horses. At the end of the day, your horse’s health and welfare is worth becoming as educated as possible about gastric health, and how to best manage it.
IMPORTANT SAFETY INFORMATION: GASTROGARD safety has not been determined in pregnant or lactating mares. For use in horses and foals 4 weeks of age and older. Keep this and all drugs out of the reach of children. In case of ingestion, contact a physician. Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
For prescribing information, click here.
References:
1 Sykes BW, Hewetson M, Hepburn RJ, Luthersson N, Tamzali Y. European College of Equine Internal Medicine Consensus Statement — Equine Gastric Ulcer Syndrome in Adult Horses. J Vet Intern Med. 2015 Sep-Oct;29(5):1288-99.
2 Equine Veterinary Compounding Guidelines. 2005. Available at: http://www.aaep.org/pdfs/drug_compounding_guidelines.pdf. Accessed Dec. 17, 2009.
3 Wallace M. Radiographic Evaluation of Compounded and Illegal Over-the-counter Omeprazole Products (Abstract E47). ACVIM 2017. Available at: https://images.saymedia-content.com/.image/cs_srgb/MTQ4NzczNDgxOTU2MjU1NDEx/44465-acvim_poster_fa.pdf. Accessed May 28, 2020.
4 Data on file.
5 Stanley SD, Knych HK. Comparison of Pharmaceutical Equivalence for Commercially Available Preparations of Omeprazole. AAEP Proceedings. 2011;57:63.
6 Nieto JE, Spier S, Pipers FS, Stanley S, Aleman MR, Smith DC, Snyder JR. Comparison of Paste and Suspension Formulations of Omeprazole in the Healing of Gastric Ulcers in Racehorses in Active Training. J Am Vet Med Assoc. 2002 Oct 15;221(8):1139-43.
7 GASTROGARD product label.
8 Animal Health Institute and American Veterinary Medical Association and American Veterinary Distributors Association. Veterinary Compounding. Available at: http://www.aaep.org/siteadmin/modules/page_editor/images/files/AHI%20Compounding.pdf. Accessed Feb. 9, 2012.
Minimizing serious fractures of the racehorse fetlock
By VA Colgate, PHL Ramzan & CM Marr
Minimizing serious fractures of the racehorse fetlock
In March 2020, a symposium was held in Newmarket, UK, aiming to devise measures which could be used internationally to reduce the risk of catastrophic fracture associated with the fetlock joint. The meeting was supported by the Gerald Leigh Charitable Trust, the Beaufort Cottage Charitable Trust and the Jockey Club with additional contributions from a number of industry stakeholders. On the first day a panel of international experts made up of academic professors, Chris Whitton (Melbourne, Australia), Sue Stover (Davis, California), Chris Kawcak (Colorado), Tim Parkin (Glasgow) and Peter Muir (Wisconsin); experienced racehorse clinicians, Ryan Carpenter (Santa Anita) and Peter Ramzan (Newmarket); imaging experts, Sarah Powell (Newmarket) and Mathieu Spriet (Davis, California); and vets with experience in racing regulatory bodies, Scott Palmer (New York) and Chris Riggs (Hong Kong) joined forces to discuss risk assessment protocols, particularly those based on imaging features which might indicate increased risk of imminent fracture. This was followed by a wider discussion with a diverse invited audience of veterinary and industry stakeholders on how our current knowledge of fracture pathophysiology and risk factors for injury could be used to target risk assessment protocols. A report of the workshop outcomes was recently published in Equine Veterinary Journal.
The importance of risk reduction
With the ethics of the racing industry now in the public spotlight, there is recognition that together veterinary and horseracing professionals must strive to realise an improvement in equine injury rates. Intervention through risk profiling programmes, primarily based on training and racing metrics, has a proven track record; and the success of a racing risk management program in New York gives evidence that intervention can and will be successful.
The fetlock of the thoroughbred racehorse is subjected to very great loads during fast work and racing, and over the course of a training career this can result in cumulative changes in the bone underlying the articular cartilage (‘subchondral’ bone) that causes lameness and may in some circumstances lead to fracture. Fracture propagation involving the bones of the fetlock (cannon, pastern or proximal sesamoid bones) during fast work or racing can have catastrophic consequences, and while serious musculoskeletal injuries are a rare event when measured against race starts, there are obviously welfare and public interest imperatives to reduce the risk to racehorses even further. The dilemma that faces researchers and clinicians is that ‘fatigue’ injuries of the subchondral bone at some sites within the fetlock can be tolerated by many racehorses in training while others develop pathology that tips over into serious fracture. Differentiating horses at imminent risk of raceday fracture from those that are ‘safe’ to run has not proven particularly easy based on clinical grounds to date, and advances in diagnostic imaging offer great promise.
Profiling to inform risk assessment
Risk profiling examines the nature and levels of threat faced by an individual and seeks to define the likelihood of adverse events occurring. Catastrophic fracture is usually the end result of repetitive loading, but currently there are no techniques that can accurately determine that a bone is becoming fatigued until some degree of structural failure has actually occurred. However, diagnostic imaging has clear potential to provide information about pathological changes which indicate the early stages of structural damage.
Previous research has identified a plethora of epidemiological factors associated with increased risk of serious catastrophic musculoskeletal injury on the racetrack. These can be distilled into race, horse and management-related risk factors that could be combined in statistical models to enable identification of individual horses that may be at increased risk of injury.
In North America, the Equine Injury Database compiles fatal and non-fatal injury information for thoroughbred racing in North America. Since 2009, equine fatalities are down 23%; and important risk factors for injury have been identified, and this work has driven ongoing improvement.
The problem with all statistics-based models created so far for prediction of racehorse injury is that they have limited predictive ability due to the low prevalence of racetrack catastrophic events. If an event is very rare, and a predictive tool is not entirely accurate, many horses will be incorrectly flagged up as at increased risk. At the Newmarket Fetlock workshop, Prof Tim Parkin shared his work on a model which was based on data from over 2 million race starts and almost 4 million workout starts. Despite the large amount of data used to formulate the model, Tim Parkin suggested that if we had to choose between two horses starting in a race, this model would only correctly identify the horse about to sustain a fracture 65% of the time. Furthermore, the low prevalence of catastrophic injury means it will always be difficult to predict, regardless of which diagnostic procedure is employed.
Where do the solutions lie?
A radiograph showing a racing thoroughbred’s fetlock joint. The arrow points to a linear radiolucency in the parasagittal groove of the lower cannon bone—a finding that is frequently detectable before progression to serious injury.
One possible strategy to overcome the inherent challenge of predicting a rare event involves serial testing. Essentially with this approach, a sequence of tests is carried out to refine sub-populations of interest and thus improve the predictive ability of the specific tests applied. An additional consideration in the design of any such practical profiling system would have to be the ability to speedily come to a decision. For example, starting with a model based on racing and training metrics such as number of starts and length of lay-off periods, as well as information about the risk associated with any particular track or racing jurisdiction, entries could be screened to separate those that are not considered to be at increased risk of injury from a smaller sub-group of horses that warrant further evaluation and will progress to Phase 2. The second phase of screening would be something relatively simple. Although not yet available, there is hope that blood tests for bone biomarkers or genetic profiles could be used to further distil horses into a second sub-group. This second sub-group might then be subjected to more detailed veterinary examination, and from that a third sub-group, involving a very small and manageable number of horses flagged as potentially at increased risk, would undergo advanced imaging. The results of such diagnostic imaging would then allow vets to make evidence-based decisions on whether or not there is sufficient concern to prompt withdrawal of an individual from a specific race from a health and welfare perspective. Of course there are other considerations which limit the feasibility of such a system, including availability of diagnostic equipment and whether or not imaging can be quickly and safely performed without use of sedation or other drugs, which are prohibited near to a race start.
Diagnostic techniques for fetlock injury risk profiling
Currently there is no clear consensus on the interpretation of images from all diagnostic imaging modalities, and important areas of uncertainty exist. Although a range of imaging modalities are available, each has its own strengths and weaknesses, and advances in technology currently outstrip our accumulation of published evidence on which to base interpretation of the images obtained.
Interpretation is easy when the imaging modality shows an unequivocal fracture such as a short fissure in a cannon bone. Here the decision is simple: the horse has a fracture and must stop exercising. Many cases, however, demonstrate less clearly defined changes that may be associated with bone fatigue injury.
Currently radiography remains the most important imaging modality in fetlock bone risk assessment. With wide availability and the knowledge gained by more advanced imaging techniques refining the most appropriate projections to use; radiography represents a relatively untapped resource that through education of primary care vets could immediately have a profound impact on injury mitigation. The most suitable projection with which to detect prodromal condylar fracture pathology in the equine distal limb is the flexed dorsopalmar (forelimb) or plantarodorsal (hindlimb) projection. On this projection, focal radiolucency in the parasagittal groove, whether well or poorly defined, with or without increased radio-opacity in the surrounding bone, should be considered representative of fracture pathology unless evidence from other diagnostic imaging modalities demonstrates otherwise.
Computed Tomography (CT) excels at identification of structural changes and is better than radiography at showing very small fissures in the bone. However, additional research is needed to determine specific criteria for interpretation of the significance of small lesions in the parasagittal groove with respect to imminent risk of serious injury. There are good indications that fissure lesion size and proximal sesamoid bone volumetric measurements have the potential to be useful criteria for prediction of condylar and proximal sesamoid bone fractures respectively. With technological advancement, it is likely that CT will be more widely used in quantitative risk analysis in the future.
Magnetic Resonance Imaging (MRI) has the ability to detect alterations in the fluid content of bones, which allows assessment of acute, active changes. Indeed standing, low-field MRI has been shown to be capable of detecting bone abnormalities not readily identifiable on radiography and has been successfully used for injury mitigation in racehorse practice for some time. However, when used for evaluation of cartilage and subchondral bone lesions, there is a relatively high likelihood of false positive results.
PET is the most recent advance in diagnostic imaging. It is being developed in California and, when combined with CT, provides information on bone activity and structure. In these three images of the same fetlock, from different aspects, the orange spots indicate increased activity in the proximal sesamoid bone, which is a potential precursor to more serious injury.
Image courtesy of Dr M. Spriet, University of California, Davis.
CLICK HERE to return to issue contents
ISSUE 57 (PRINT)
$6.95
ISSUE 57 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - ONLY $24.95
Bleeders - facts- fiction - future - treatment - of exercise-induced haemorrhages
By Dr. David Marlin
We are now approaching half a century since Bob Cook pioneered the use of the flexible fibreoptic endoscope, which allowed examination of the respiratory tract in the conscious horse. One of the important outcomes of this technique was that it opened the door to the study of “bleeding” or exercise-induced pulmonary hemorrhage (EIPH).
But nearly 50 years on the irony is perhaps that whilst we have become good at describing the prevalence of EIPH and some of the factors that appear to increase the severity of EIPH within individual horses, we still lack a clear understanding of the condition and how to manage it.
I use the term manage rather than treat or prevent as our knowledge of EIPH must show us that EIPH cannot be stopped entirely; it is a consequence of intense exercise. The other irony is that in the past 50 years, by far the majority of research into the management of EIPH has focused on the use of the diuretic furosemide. Whilst we have good evidence from controlled studies that furosemide reduces the severity of EIPH on a single occasion, we still lack good evidence to suggest that furosemide is effective when used repeatedly during training and or racing; and there is also evidence to the contrary. Let’s review some basic facts about EIPH, which should not be contentious.
• EIPH is the appearance of blood in the airways associated with exercise.
• EIPH occurs as a result of moderate to intense exercise. In fact, EIPH has been found after trotting when deep lung wash (bronchoalveolar lavage or BAL) is done after exercise.
• EIPH most often involves the smallest blood vessels (capillaries) but can sometimes and less commonly be due to the rupture of larger blood vessels.
• The smallest blood vessels are extremely thin. Around 1/100th the thickness of a human hair. But this extremely thin membrane is also what allows racehorses such as Thoroughbreds, Standardbreds and Arabs to use oxygen at such a high rate and is a major reason for their athleticism.
• EIPH is a progressive condition. The chance of seeing blood in the trachea after exercise increases with time in racing.
• EIPH is variable over time, even when horses are scoped after the same type of work.
• If you ‘scope a horse after three gallops in a row, you can expect to see blood in the trachea on at least one occasion.
• EIPH damage to the lungs starts at the back and top, and over time moves forward and down and is approximately symmetrical.
• Following EIPH the lung becomes fibrotic (as scar tissue), stiffer and does not work as well. The iron from the blood is combined with protein and stored permanently in the lung tissue where it can cause inflammation.
• High blood pressure within the lung is a contributing factor in EIPH. Horses with higher blood pressure appear to suffer worse EIPH.
• There is also evidence that upper airway resistance and breathing pattern can play a role in EIPH.
• Airway inflammation and poor air quality may increase the severity of EIPH within individual horses.
• Increasing severity of EIPH appears to have an increasing negative effect on performance.
• Visible bleeding (epistaxis) has a very clear and marked negative effect on performance. In order to make progress in the management of EIPH (i.e., to minimize the severity of EIPH in each individual), there are certain steps that trainers can take based on the information we have to date. These include:
• Ensuring good air quality in stables • Regular respiratory examination and treatment of airway inflammation • Reduced intensity of training during periods of treatment for moderate to severe airway inflammation
• Extended periods of rest and light work with a slower return to work for horses following viral infection
• Addressing anything that increases upper airway resistance (e.g., roaring, gurgling)
• Avoiding intense work in cold weather
• Avoiding extremes of going
• Limiting number of training days in race preparation and increasing interval between races.
FUTURE OPPORTUNITIES IN UNDERSTANDING AND MANAGING EIPH
We have to accept EIPH as a normal consequence of intense exercise in horses. Our aim should be to reduce the severity to a minimum in each individual horse. However, there are areas in which we still need a much greater scientific understanding.
What actually causes the capillaries to leak or rupture?
If you ask any vet, scientist or informed trainer what is the cause of EIPH, they will give the phrase “pulmonary capillary stress-failure”. But this is simply a description of what happens—NOT an explanation or a mechanism. EIPH and pulmonary capillary stress-failure are both descriptions of what’s happening. We know high blood pressure makes the capillaries stiff. But what makes them actually rupture? A balloon filled with water may be distended and under a lot of stress. But a pin prick will actually make it burst. The pin is the cause.
Assessing EIPH
At present the most common way to assess the severity of EIPH in horses in training and racing is by ‘scoping 30-40 minutes after exercise and scoring the amount of blood in the trachea. This is a crude method, and when we see a horse that has a score of 1 after one gallop and a 3 after the next gallop, we don’t know whether this is due to differences in how quickly the blood has moved from the periphery of the lung into the trachea or due to a true difference in the amount of bleeding. We know our ‘scoping scores vary from gallop to gallop; we just don’t know why.BAL (deep lung wash) is not the answer either. It will pick up blood when there is none to be seen in the trachea (i.e., it’s a more sensitive technique), but with BAL we are looking atrelatively small areas of the lung. What we need is a technique that will allow us to image the whole lung and map the blood that is in the airways and not in the blood vessels so we can assess volume and distribution of hemorrhage.
Furosemide is not the answer
A number of well-conducted and well-written scientific studies have shown conclusively that furosemide is effective in reducing the severity of EIPH in individual horses when used ONE time! We lack convincing studies that prove furosemide works as well when used one to two times a week for two to three months. In fact, several studies suggest that furosemide becomes less effective with regular use, such as the return to previous performance of horses after initial racing and improved performance on furosemide. In human medicine, repetitive administration of furosemide induces short-term (braking phenomenon, acute diuretic resistance) and longterm (chronic diuretic resistance) tolerance (i.e., if you give the same dose repeatedly, the body becomes tolerant and you get less and less urine production). A study in horses from Michigan State University in 2017 showed horses develop tolerance to furosemide. Why, when we have had nearly 50 years of research into EIPH with more published studies devoted to furosemide than any other aspect, do we still not know if furosemide is effective when used on a regular basis?
Is EIPH really blood?…
CLICK HERE to return to issue content
BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 56 (PRINT)
$6.95
ISSUE 56 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - PRINT & ONLINE - ONLY $24.95
Bowed tendons - different treatment options - new ultrasound technology - ultrasound tissue characterization
By Sarah Plevin
Overstrain injuries to the superficial digital flexor tendon (SDFT) are among the most common musculoskeletal injuries for all athletic equine disciplines but account for a significant amount of wastage in the Thoroughbred (TB) racehorse.
Treatment options for such ‘bowed tendons’ are many and varied, but all have a couple of things in common: time out of training; expense and no guarantee of success.
It makes sense then, that prevention of injury should always be the goal, and failing that, a method to optimally guide rehabilitation is needed.
Unfortunately, limitations of current imaging diagnostics have restricted their use for accurately monitoring the tendon.
A new ultrasound technology, however, called ultrasound tissue characterization, may get us one step closer to achieving the goals of injury prevention and optimal rehabilitation.
What would the ideal tendon imaging modality allow us to do?
Monitor the effects of exercise on the tendon
Early detection of overstrain injuries
Be able to stage the lesion, i.e., determine the level of degenerative change within the tendon structure
Fine-tune therapy
Guide rehabilitation
Why are tendon injuries so tricky?
Figure 1: Functionally normal healthy aligned tendon bundles.
A normal healthy tendon is made from aligned organized tendon bundles. (Figure 1) Deterioration of this structure ranges on a spectrum from complete disruption (core lesion) to more minor changes, but all affect the ability of the tendon to function optimally.
Degenerative changes within the tendon matrix are not uniform—meaning that not all overstrain injuries to the SDFT are represented by the same level of deterioration or structural change, so there is not a one-size-fits-all pathology or diagnosis, and therefore there cannot be a cure-all treatment.
Most tendon injuries have a sneaky onset with tendon degeneration developing initially without clinical signs, so problems start without you or your horse even knowing about them. Often by the time you realize there is a problem, tendon matrix degradation has already begun.
Staging the structural integrity of the tendon or classifying the extent of structural deterioration present is, therefore, imperative—not only for optimal therapy selection and appropriate rehabilitation guidance but also if prevention of injury is ever to be achieved.
Why isn’t conventional ultrasound enough?
Unfortunately, although conventional ultrasound has historically been used to evaluate equine tendon, limitations have restricted its ability to accurately monitor tendon structure, predict injury or guide rehabilitation.
Clinical improvement is usually not accurately correlated with changes in imaging status using conventional ultrasound, especially in the later stages of healing with conventional ultrasound not demonstrating enough sensitivity to determine the type of tendon tissue under investigation.
So, while regular ultrasound can easily demonstrate the presence of a core lesion when it first appears, by about two months post injury, its capacity to provide information regarding the health of the tendon is limited. Because of its inability to interpret the integrity of the underlying tendon structure accurately, along with inconsistencies in imaging, reliance on operator skills and the inherent lack of ability of a 2D conventional ultrasound image to fully decipher a 3D tendon structure, its ability to reliably evaluate and monitor the SDFT following the initial acute period is severely restricted.
What is ultrasound tissue characterization?
Ultrasound tissue characterization is a relatively new technique intended to alleviate some of the problems encountered with conventional ultrasound by improving objective tendon characterization. It does this by providing a 3D reconstruction of the tendon and by classifying and then quantifying tendon tissue into one of four color-coded echo types based on the integrity of the tendon structure.
It can assess in detail the structural integrity of the tendon; it can discriminate a variety of pathological states and is sensitive enough to detect the effect of changing loads on the tendon within days.
What do the colors mean? (Figure 2)
Figure 2: Color-coded ultrasound tissue characterization echo types represent the stability of echo pattern over contiguous images related to tendon matrix integrity.
Green (type 1 echoes) are normal, well-aligned and organized tendon bundles, and at least 85-90% of this echo type should be found in a healthy tendon (SDFT). Blue (type 2 echoes) are areas of wavy or swollen tendon bundles. They can represent remodeling and adapting tendon or inferior repair. Red (type 3 echoes) represents fibrillar tissue (the smaller basic unit or building block of tendon). This echo type can represent partial rupture of the tendon where they reflect breakdown of normal structure or they can represent initial healing as the tendon begins to rebuild. Black (type 4 echoes) are areas of cells or fluid and represent core lesions where no normal tendon tissue exists.
How is ultrasound tissue characterization currently used?
The aim of ultrasound tissue characterization is not to replace conventional ultrasound but on the contrary, it is recommended to perform an evaluation with both conventional B mode ultrasound and ultrasound tissue characterization to achieve a complete picture of tendon health.
Currently it is used successfully in elite human athletes such as NBA and soccer players to monitor the health of their tendons (Achilles tendon and patellar tendons) and to guide exercise regimens post injury.
Figure 3: Ultrasound tissue characterization tracker frame with attached ultrasound probe.
In the equine field, it is used in elite sport horses as part of routine maintenance evaluations to direct exercise, to monitor tendon health and guide rehabilitation following an injury.
How does it work?
It consists of a standard linear ultrasound probe mounted onto a motorized tracking device (Figure 3). Due to the sensitivity of this equipment, the limbs should be clipped in order to obtain good quality images.
The probe moves non-invasively and automatically down the tendon from top to bottom over a 12-cm scanning distance (see Introphoto) …
CLICK HERE to return to issue contents
BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 56 (PRINT)
$6.95
ISSUE 56 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - PRINT & ONLINE - ONLY $24.95
PET scanning - reduces catastrophic fractures - latest advance in equine imaging - designed to image horse legs
By Mathieu Spriet, Associate Professor, University of California, Davis
Santa Anita Park, the iconic Southern California racetrack, currently under public and political pressure due to a high number of horse fatalities during the 2019 season, announced in December 2019 the installation of a PET scanner specifically designed to image horse legs. It is hoped that this one-of-a-kind scanner will provide information about bone changes in racehorses to help prevent catastrophic breakdowns.
What is PET?
Figure 1: The first equine PET was performed in 2015 at the University of California Davis on a research horse laid down with anesthesia. The scanner used was a PET prototype designed for the human brain (piPET, Brain- Biosciences Inc., Rockville, MD).
PET stands for positron emission tomography. Although this advanced form of imaging only recently became available for horses, the principles behind PET imaging have been commonly used at racetracks for many years. PET is a nuclear medicine imaging technique, similar to scintigraphy, which is more commonly known as “bone scan”. For nuclear imaging techniques, a small dose of radioactive tracer is injected to the horse, and the location of the tracer is identified with a camera in order to create an image. The tracers used for racehorse imaging are molecules that will attach to sites on high bone turnover, which typically occurs in areas of bone subject to high stress. Both scintigraphic and PET scans detect “hot spots” that indicate—although a conventional X-ray might not show anything abnormal in a bone—there are microscopic changes that may develop into more severe injuries.
Development of PET in California
The big innovation with the PET scan is that it provides 3D information, whereas the traditional bone scan only acquires 2D images. The PET scan also has a higher spatial resolution, which means it is able to detect smaller changes and provide a better localisation of the abnormal sites. PET’s technological challenge is that to acquire the 3D data in horses, it is necessary to use a ring of detectors that fully encircles the leg.
The first ever equine PET scan was performed at the School of Veterinary Medicine at the University of California in 2015. At the time, a scanner designed to image the human brain was used (PiPET, Brain-Biosciences, Inc.). This scanner consists of a horizontal cylinder with an opening of 22cm in diameter. Although the dimensions are convenient to image the horse leg, the configuration required the horse be anesthetised in order to fit the equipment around the limb.
The initial studies performed on anesthetised horses with the original scanner demonstrated the value of the technique. A first study, published in Equine Veterinary Journal, demonstrated that PET showed damage in the equine navicular bone when all other imaging techniques, including bone scan, MRI and CT did not recognise any abnormality.
A pilot study looking at the racehorse fetlock, also published in Equine Veterinary Journal, showed that PET detects hot spots in areas known to be involved in catastrophic fractures.
Figure 2: These are images from the first horse image with PET. From left to right, PET, CT, MRI, and bone scan. The top row shows the left front foot that has a severe navicular bone injury. This is shown by the yellow area on the PET image and abnormalities are also seen with CT, MRI and bone scan. The bottom row is the right front foot from the same horse, the PET shows a small yellow area that indicates that the navicular bone is also abnormal. The other imaging techniques however did not recognize any abnormalities.
This confirmed the value of PET for racehorse imaging, but the requirement for anesthesia remained a major barrier to introducing the technology at the racetrack. To overcome this, LONGMILE Veterinary Imaging, a division of Brain-Biosciences Inc, in collaboration with the University of California Davis, designed a scanner which could image standing horses. To do this, the technology had to be adapted so that the ring of detectors could be opened and positioned around the limb.
With the support from the Grayson Jockey Club Research Foundation, the Southern California Equine Foundation and the Stronach Group, this unique scanner became a reality and, after the completion of an initial validation study in Davis, the scanner was installed at Santa Anita Park in December 2019.
Figure 3: The two images on the left are bone scan images from a 4-year-old Thoroughbred racehorse. The images on the right are 3D projection of PET images of the same fetlock. The bone scan revealed an abnormality at the bottom of the cannon bone. The PET scan confirmed this abnormality and helped better localize it. In addition, several other abnormalities were found on the PET scan in the sesamoid bones.
PET at the racetrack
The new PET scanner has been used to image the equine limb from the foot to the knee. The current main focus at the racetrack is fetlock imaging, as the majority of catastrophic breakdown in racehorses affects this area. The UC Davis pilot study highlighted the value of PET for detecting abnormalities in the proximal sesamoid bones—the two small bones at the back of the cannon bone—that are commonly involved in catastrophic fractures. Previous necropsy research on horses which suffered breakdowns has shown that changes can be present in the bones prior to the development of major injuries. The goal of the Californian PET project is to detect these warning signs in order to avoid training and racing horses at high risk for catastrophic breakdown.
Alternative imaging techniques
Other imaging techniques are available for examining equine bone. Scintigraphic bone scans are doing an excellent job at detecting stress fractures of the humerus or tibia, and this has helped markedly decrease catastrophic injuries in these areas. Bone scan is also used for fetlocks; but “hot fetlocks” are common on bone scan, and the lower resolution 2D images often do not allow to truly determine whether horses are at high risk of fractures or have normal bone adaptation to training.
Figure 4: The MILE-PET scanner (LONGMILE Veterinary imaging, Rockville, MD) is the first PET scanner specifically designed to image standing horses. An openable ring of detectors allows easy positioning and safe scanning.
MRI is used for fetlock imaging too, and MRI scanners designed for imaging standing horses have been available for over 15 years. Several large racing centers are equipped with such scanners, and MRI excels in particular at detecting changes in the cannon bone that precede condylar fractures. MRI can detect areas of bone densification, or even accumulation of fluid in the bone, typically indicative of microtrauma that can weaken the bone.
Computed tomography (CT) has also recently been used for standing imaging of the fetlock. At the moment, there are a few centers equipped with a CT scanner allowing standing fetlock imaging, but they are only available at, for example, New Bolton Center, Pennsylvania - USA, and the University of Melbourne, Australia. CT uses X-rays to create 3D images. Similar to MRI, CT can detect areas of bone densification or areas of bone loss. …
CLICK HERE to return to issue contents
BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 56 (PRINT)
$6.95
ISSUE 56 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - PRINT & ONLINE - ONLY $24.95
Colic – effects of inflammation
By Dr. Zofia Lisowski
Overview of colic
Colic is a term used to describe the display of abdominal pain in a horse. It is the most common emergency in horses with four to ten out of every 100 horses likely to experience at least one episode of colic each year. It is also the single most common cause of equine mortality. In the U.S., one study showed that Thoroughbreds were more likely to develop colic1 than other breeds. It is of great welfare concern to horse owners, and with the estimated costs associated with colic in the U.S. exceeding $115 million per year2 and the average cost of a horse undergoing colic surgery requiring a resection being approximately $8,3703, it is also a significant economic issue for horse owners.
Horses with abdominal pain show a wide range of clinical signs, ranging from flank watching and pawing the ground in mild cases, to rolling and being unable to remain standing for any significant period of time in more severe cases. There are numerous (over 50) specific causes of colic. In general, colic occurs as a result of disruption to the normal function of the gastrointestinal tract. This may be attributable to mechanical causes such as an obstruction (constipation), distension (excess gas) or a volvulus (twisted gut). It may also have a functional cause, whereby the intestine doesn’t work as normal in the absence of an associated mechanical problem; for example, equine grass sickness is associated with a functional derangement of intestinal motility due to loss of nerves within the intestine.
Management of colic depends on the cause and can necessitate either a medical or surgical approach. Most horses with colic will either improve spontaneously or with simple medical treatment alone; however, a significant proportion may need more intensive medical treatment or surgery. Fortunately, due to improvements in surgical techniques and post-operative management, outcomes of colic surgery have improved over the past few decades with up to 85% of horses surviving to discharge. Crucially for the equine Thoroughbred racehorse population, several studies focused on racehorses that had undergone colic surgery and survived to discharge, reporting that 63-73% returned to racing. Furthermore, surgical treatment did not appear to negatively impact athletic performance. A similar finding was also seen in the general sport horse population.
Despite significant advancement in colic surgery per se, complications following surgery can have a significant impact on post-operative survival and return to athletic function. Common post-operative complications include:
Complications at the site of the incision (surgical wound)
Infection: Infections at the surgical incision site are relatively common. Antibiotics are usually administered before surgery and after surgery. Infections are not normally severe but can increase treatment costs. Horses that develop infections are at greater risk of developing an incisional hernia.
Hernia: Incisional hernias occur when the abdominal wall muscles fail to heal, leaving a “gap.” Hernia size can vary from just a few centimeters, up to the full length of the incision. Most hernias will not require further treatment; but in more severe cases, further surgery may be required to repair the hernia.
Complications within the abdomen
Hemoperitoneum: A rare complication where there is blood within the abdomen from bleeding at the surgical site.
Anastomosis complications: The anastomosis site is where two opposing ends of intestine that have been opened are sutured back together again. It is important that at this site no leakage of intestinal contents occurs. Leakage or breakdown at this site can lead to peritonitis, which is inflammation or infection within the abdominal cavity and is a potentially life-threatening complication.
Adhesions: Scar tissue can form within the abdomen following abdominal surgery. Occasionally this may cause further colic episodes.
Further colic episodes
Further colic episodes can occur following surgery. These can occur days to months following discharge.
Endotoxemia
In some rare cases, horses may develop sepsis in response to toxins released by damaged intestines.
Diarrhea
This is a rare complication. It can develop as a result of infections with C. difficile or Salmonella. As a consequence, some horses may need to be treated in isolation to ensure infection doesn’t spread to other horses or humans.
Post-operative ileus
Post-operative ileus is one of the potential post-operative complications that can lead to a significant increase in hospital stay duration, increased treatment costs and is also associated with reduced survival rates. Post-operative ileus is a condition that affects the muscle function in the intestinal wall. The intestine is a long tube-like structure that has a muscular wall throughout its entire length from the esophagus to the anus. The function of this muscle is to contract in waves to mix and move food along the length of the intestinal tract, within which digestion occurs and nutrients are absorbed, terminating in the excretion of waste material as feces. In post-operative ileus these contractions stop and thus intestinal contents are not moved throughout the intestinal tract. In most cases, it is transient and lasts for up to 48 hours following surgery; however, in some cases it can last longer. A build-up of fluid develops within the intestine as a result of the lack of propulsion. This stretches the intestines and stomach, resulting in pain and the horse’s inability to eat. Unlike humans, the horse is unable to vomit; consequently, this excess fluid must be removed from the stomach by other means, otherwise there is a risk of the stomach rupturing with fatal consequences. Post-operative ileus may occur in up to 60% of horses undergoing abdominal surgery and mortality rates as high as 86% have been reported. Horses in which the small intestine manipulated is extensively manipulated during surgery and those that require removal of segments of intestine are at higher risk. Despite the significant risk of post-operative ileus following colic surgery in horses, there is a lack of studies investigating the mechanisms underpinning this condition in horses; consequently, the precise cause of this condition in horses is not fully known.
What causes the intestine to stop functioning? …
BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 55 (PRINT)
$6.95
ISSUE 55 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - PRINT & ONLINE - ONLY $24.95
Indiana's New "Biological Samples" Testing Law: Integrity Assured Or Invasive Overreach?
By Peter J. Sacopulos
From House Bill To Horse Law
On May 1, 2019, Governor Eric Holcomb signed Indiana House Bill 1196 into law. The statute, which took effect on July 1 of this year, directs the Indiana Horse Racing Commission (IHRC) to adopt a variety of new rules and procedures governing horse racing within the state.
Governor Holcomb and Indiana State Representative Bob Cherry, who introduced HB 1196 to the legislature, are Republicans. However, the bill enjoyed broad bipartisan support—a rarity in current American politics. In fact, the final version sailed through both chambers, receiving not a single “nay” vote in the House and a mere three “nays” in the Senate.
HB 1196 is something of an equine regulatory smorgasbord. Examples of its provisions include officially changing references to the IHRC’s “secretary” to “executive director,” altering the way breed advisory committee members are appointed, specifying that certain funds be directed to the Indiana-sired horses program, and the creation of new privacy protections to guard the personal information required on license applications.
Items like these, as well as several others included in HB 1196, are unlikely to cause ripples within the racing community. However, the new law also includes provisions designed to enhance and expand the Commission’s ability to detect, police and reduce the use of banned substances. And while this is undoubtedly a worthwhile cause, a two-word term used in the drug-testing language of HB 1196 has the potential to negatively impact horses and trainers for years to come, with consequences that may spread well beyond the borders of Indiana.
Two Words, Too Broad?
The two-word term is “biological sample.” It is legally defined in the statue as follows:
“Biological sample” refers to any fluid, tissue or other substance obtained from a horse through an internal or external means to test for foreign substances, natural substances at abnormal levels, and prohibited medications. The term includes blood, urine, saliva, hair, muscle tissue collected at a necropsy, semen, and other substances appropriate for testing as determined by the commission.
This definition goes well beyond the longstanding blood, saliva, urine, and more recently, hair, samples routinely collected from Thoroughbred competitors for analysis. It is also disturbingly open-ended. Indeed, the phrase: “…and other substances appropriate for testing as determined by the commission…” is a definition that is essentially wide open, providing the IHRC staff the power to define or redefine a “biological sample.”
While there was discussion and temporary agreement to limit the use of biological samples to necropsy purposes, that limitation was removed from the final version of the bill that was signed into law and became effective July 1, 2019. Therefore, the Commission Staff is authorized and may elect to take muscle tissue, and other biological samples, from live animals as it deems and determines necessary and appropriate. This rule and its definition of biological sample establishes a new frontier of testing.
Is This Risk Really Necessary?
One of the primary concerns and positions advanced in opposition to allowing biological samples to be taken from live animals is the risk of injury.
Taking saliva and hair samples from a Thoroughbred is painless and easy. And anyone who has ever been around horses knows that they are more than happy to provide all the urine you could ever want! Drawing blood from a horse is only slightly more difficult and rarely involves the use of a local anesthetic.
However, taking “…any fluid, tissue or other substance… through an internal or external means…” is another matter entirely. It opens the door to far more invasive collection techniques that carry far greater risks for horses than blood, saliva, urine or hair sampling. To be clear, I am referring to biopsies.
A biopsy is the removal and examination of cells or tissue from a living being for the purposes of testing and examination. Any biopsy carries risk of injury or infection. Taking a biopsy from a horse may be as simple as a skin sample from the withers or tissue from the lining of the mouth, or as difficult as removing material from the teeth or the interior of the eye; or from internal organs such as the heart, lungs, liver, intestine or colon. In the latter examples, a biopsy becomes a complex medical procedure. A procedure performed on a large, valuable animal requires sedation and may require general anesthesia to facilitate tissue collection.
Sedating a horse is serious business. Sedatives and anesthetics carry significant risks, even when administered with care by skilled equine veterinary professionals. Those risks include allergic reactions, collapse, excitement, cardiac arrest, medical injury and post-anesthetic colic.
TO READ MORE - BUY THIS ISSUE IN PRINT OR DOWNLOAD
ISSUE 54 (PRINT)
$6.95
ISSUE 54 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
Four issue subscription - PRINT & ONLINE - ONLY $24.9
Skin deep - overcoming barriers for effective transdermal drug delivery
By Professor Roger Smith
Ancient art, modern science
One shared medicinal practice among disparate ancient societies was the application of primitive ointments to the skin to treat almost all and any ailments. A vast plethora of poultices and plasters have been described, including in Babylonian and Greek medicine texts1 among others, suggesting that the magical health-restoring powers of ointments were well-recognized to traverse the skin. Thus, it was no coincidence that the skin was the preferred therapeutic route over surgical (and oral) intervention since the former method was likely to result in reduced mortality rates compared to the latter; undoubtedly an important consideration, given that the top ancient physicians were likely charged with the health of the royal courts.
Although the art of transdermal delivery of medicines dates back millennia, it is only in more recent times that the science of transdermal drug delivery in man has advanced significantly. The choice of modern drugs for topical applications is, however, relatively limited compared to the seemingly infinite choice available for oral delivery. This is perhaps not surprising since the gut is an organ that has evolved with the main purpose of absorbing food (chemicals when it comes to it) whereas the skin, despite being the largest organ, has evolved primarily as a protective layer to prevent desiccation of underlying tissues and to keep out harmful environmental chemicals. As this includes medicinal drugs, the pursuit of transdermal administration would appear, at first sight, to be an illogical choice. However, there are several compelling reasons why transdermal delivery routes are an important alternative to pills, injections or inhalation routes:
It avoids poor absorption after oral ingestion—especially in animals, the absorption of a drug can vary between the omnivore (e.g., human) and herbivore (e.g., horse) stomach.
It avoids first-pass effect where the blood circulation from the gut passes through the liver to remove absorbed drugs.
It can reduce systemic drug levels to minimize adverse effects.
The design of sustained release formulations overcomes the frequent dosing necessitated by oral and injectables to achieve constant drug levels.
It enables ease and efficacy of drug withdrawal.
Transdermal drug delivery is painless and non-invasive, thereby potentially allowing longer treatment when daily injection is unacceptable or impractical.
It has the potential to target local administration such as for the treatment of flexor tendon disease because the tendons are subcutaneous.
Challenges for transdermal drug applications
The skin is made up of three key layers: the epidermis, dermis and hypodermis and the water-attracting (hydrophilic) or water-repelling (hydrophobic) properties within each raise unique challenges for topical or transdermal drug applications.
Topical applications, such as insect repellents and sunscreen creams, target the surface of the skin or deliver a drug locally such as for the control of inflammation (insect bite or reaction to an allergen). In contrast the aim of transdermal, or subcutaneous, applications are to deliver the drug deeper to either an adjacent organ, or, more commonly, to the blood circulation as an alternative to oral or needle routes to reach distant organs. The main barrier to local or transdermal delivery is the outermost layer of the skin, called the stratum corneum in the epidermis. This consists of dead skin cells, the corneocytes, that combine with lipid bilayers into a tightly packed “bricks-and-mortar” layer that form alternating hydrophilic (the water rich corneocytes) and hydrophobic (lipid bilayer) regions (figure 1). The stratum corneum therefore not only forms a mechanically robust layer but also presents a challenge in designing drugs with chemical properties that can negotiate their way into and through these contrasting hydrophobic and hydrophilic environments to reach the lower region of the epidermis. The epidermis consists of living skin cells but has no blood vessels for the drug to diffuse into, so instead the drug must penetrate further to the dermis where it can finally enter the bloodstream or the subcutaneous layers.
Routes for drugs through the skin
Most transdermal drugs are designed so that they diffuse through the skin in a passive fashion. The routes for drug can be through the skin cells (transcellular), around them (intercellular) or using the skin components—hair follicles, sweat glands and sebaceous glands (produce lipids)—to bypass the stratum corneum (so-called “appendageal” routes).
Transcellular route: Drugs pass through the corneocytes of the stratum corneum rather than the lipid ‘mortar’ that surrounds them. However, the drug has to exit the cell to enter the next corneocyte and therefore through the skin. It means that it has to encounter the external hydrophobic environment between the cells multiple times as it moves through the alternating cell and lipid layers of the epidermis. Drugs therefore have to have balanced hydrophilic and hydrophobic properties to enable this to happen.
Intercellular route: The drug predominantly diffuses through the lipid rich “mortar” around the corneocytes of the epidermis. This lipid matrix can form a continuous route through the epidermis (avoiding entering the cells), but this route has been suggested to be less efficient because it increases the distance 50-fold3 compared to the direct route through the stratum corneum due to the interdigitating brick and mortar arrangement. Again, the chemical formulation used to carry the drug is important and drugs that more readily dissolve in lipids benefit from this route.
Appendageal route: The hair, sweat glands and sebaceous glands provide a direct channel to the deep layers of the skin circumventing the hazardous barriers of the epidermis and dermis. The main challenge for this relatively easy route is that the amount of drug that can be taken up is limited by the density of hair follicles and sweat glands, although in haired animals, such as the horse, the density can be as high as 1-5% of the skin surface area. Furthermore, sweat from an active sweat gland would be travelling against the direction of drug flow, washing out the drug and its carrier and severely limit drug uptake. It is likely that all skin applications use this appendageal route as it’s unavoidable but probably more efficient for drugs that are large molecules.
TO READ MORE —
BUY THIS ISSUE IN PRINT OR DOWNLOAD -
SUMMER SALES 2019, ISSUE 53 (PRINT)
$6.95
SUMMER SALES 2019, ISSUE 53 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
PRINT & ONLINE SUBSCRIPTION
From $24.95
Fungi - the invisible health risk
By Dr. Emmanuelle van Erck
Dr. Emmanuelle van Erck, DVM, PhD, ECEIM explains her work looking at the link between the presence of fungi and lower airway inflammation
Horses are incredible athletes. Their physiology—the way their body functions—is truly fascinating. They can adapt to training at a phenomenal rate, they have massive hearts that fuel their powerful muscles and pushes them to peak speeds. So what could stop them? Oxygen, or rather the lack of it. Horses experience hypoxemia during racing, which means they enter a state of deficiency in oxygen. The reason for this deficiency is a failure of the respiratory system to effectively ventilate and adequately fuel oxygen to the muscles. Horses are obligate nasal breathers and were endowed with particularly long and narrow upper airways in relation to their body size. These factors increase the resistance to breathing. They are also constrained by the fact that they ventilate at very high rates, which does not allow for effective and rapid renewal of oxygen in the lungs. Even the fittest, best Thoroughbreds crave oxygen from mid-race onwards. So maintaining horses in optimal respiratory health is absolutely essential for them to achieve an efficient sprint and optimal performance.
Respiratory diseases are highly prevalent in horses. It is inherent to their living and working conditions. The mere fact that a horse is housed in a box increases his risk of developing airway inflammation. The content in fine dust is naturally high in a horse’s box. Closed or poorly ventilated barns further deteriorate air quality in the horse’s immediate environment. Several studies have shown that horses housed indoors are exposed not only to high amounts of organic dust and ammonia but also germs and endotoxin they produce that trigger a detrimental reaction from the immune system. The problem is that even low-grade respiratory diseases will directly affect the horse’s capacity to perform and recover from strenuous exercise.
With my colleagues, Dr. Dauviller and Dr. ter Woort, specialists in equine internal medicine, we have investigated the link between the presence of fungi and lower airway inflammation. In our ambulatory referral practice, we go out to the stables and have the opportunity not only to examine the horse but also attentively assess his environment. As we collected respiratory samples and analyzed them ourselves, we became aware that the presence of microscopic molds or fungal elements was frequently associated to lung issues. To investigate this further, we decided to systematically record clinical and environmental data and link it to our findings in the respiratory samples of the horses referred for investigation.
We collected more than 700 cases; the horses included in the study were either referred routine examinations, unexplained poor performance or respiratory symptoms such as coughing or breathing heavily during exercise. All horses had a tracheal and a bronchoalveolar lavage done, which allowed us to evaluate their level of respiratory inflammation, as well as estimate the presence of fungi within the airways. We also looked at the state of activation of fungi: if they were inert particles or if they showed signs of active proliferation. Our results were without appeal; the presence of inhaled fungi significantly and negatively affected respiratory health in horses, causing inflammation and in some cases, infection.
In this population, inflammatory airway disease (IAD) was diagnosed in 88% of cases, confirming that respiratory inflammation is very common and often under-diagnosed. Of these positive cases, 81% had evidence of fungi in their airways. The presence of fungi more than doubled the odds of having lung inflammation.
“Soaking hay and the use of haylage also came out as detrimental. On the other hand, steaming hay at high temperatures was the only means to effectively reduce risks of IAD.”
The effects of inhaled fungi have been well described in human patients but not as extensively studied in horses. The fungi constitute very small dust particles that are easily inhaled into the deeper areas of the lung. The inhaled fungi can cause inflammation of the airways by their mere presence or trigger an allergic reaction in sensitized individuals. Some fungal species will produce toxins, which can exacerbate the damages caused to the respiratory system. Furthermore, when the immune system is overloaded, some fungi will start invading the airways and cause infection. In our study, the horses that had fungi were more frequently affected with poor performance. More obvious respiratory clinical signs such as nasal discharge or coughing were not systematically observed. Some specific fungal species, such as Aspergillus type molds, were more frequently associated with lung bleeding (exercise-induced pulmonary hemorrhage).
When we looked at the link with environment, it was obvious that its hygienic quality was determinant. The use of certain types of bedding and forage were major risk factors. The use of straw bedding and dry hay constituted the highest risks of inhaling fungi and more than doubled the odds of having IAD. Soaking hay and the use of haylage also came out as detrimental. On the other hand, steaming hay at high temperatures (with a Haygain machine) was the only means to effectively reduce risks of IAD. Likewise, the use of wood shavings was protective against fungal inhalation and IAD.
Where do these fungi come from, and how can we evict them? When straw and hay are harvested, they are left to dry for a couple of days on soil. If the summer is humid, fungal content in soil is higher and the risk of contamination of the harvested hay and straw by fungi is increased. Subsequent storage of hay and straw can further promote fungal growth if temperature and humidity are favorable. Soaking hay has been shown to increase bacterial and fungal growth, whereas steaming hay effectively kills any deleterious microorganisms present in the forage, fungi included. Only hay steamers that provided high temperature steaming, right to the core of the bale, were used in the study.
Homemade incubators were exceptions but were unsafe as they did not steam at sufficiently high temperatures and served as incubators, multiplying microbial content instead of reducing it. Haylage did not come out as a protective factor probably because of the very variable quality of the bales that were used. In terms of bedding, wood shavings were the best option, as they are produced in a non-contaminated environment wrapped up in plastic. Wood also contains natural antiseptic compounds, which would prevent microbial growth.
So reducing the introduction of fungi in the stables by choosing the right forage and bedding is key to ensuring respiratory health of our horses. The problem is that the fungi we are concerned with are microscopic, meaning invisible to the human eye. Unless you have overwhelming proliferation, such as black mold on your stable walls, or hard evidence by having the environment sampled by an expert, these fungi will remain undetectable by sight or smell. It is problematic for both the equine athlete and the persons working in the stables. Once introduced in the environment (storage areas and box), fungal spores can persist for hundreds of years. To eliminate them and avoid contamination of fungal-free bedding and forage, regular thorough disinfection of the facilities is mandatory. It needs to be carefully planned, as it requires the use of chemicals that can be irritating for the horse. Once the disinfection has been effectively made, adding specific probiotics to the environment can prolong its effects. We have tested products that effectively recreate a healthy ecosystem and prevent excessive growth of potentially harmful fungi and bacteria.
There are a variety of other environmental factors that can affect horses and foster airway inflammation. These include everything from external factors such as climate and seasonal changes to internal factors, such as temperature and humidity within the stable, building configuration and ventilation, number of horses being housed. Management practices to clean can be paradoxically problematic. Human activity such as cleaning out the boxes, sweeping or the use of blowers will stir up high amounts of dust.
Our study has enabled us to prove how fungi can promote respiratory disease in horses. Horses with unexplained poor performance should be investigated for the possible implication of the respiratory system. Lung sampling can help determine if the horses have inhaled fungi and what level of inflammation is present. In addition to medical treatment, there are more global solutions that can be implemented to help affected horses through better management of the environment. Regular disinfection and the choice of adequate bedding and forage treatment can make a huge, long-term difference for the horses’ health and performance.
TO READ MORE —
BUY THIS ISSUE IN PRINT OR DOWNLOAD -
SUMMER SALES 2019, ISSUE 53 (PRINT)
$6.95
SUMMER SALES 2019, ISSUE 53 (DIGITAL)
$3.99
WHY NOT SUBSCRIBE?
DON'T MISS OUT AND SUBSCRIBE TO RECEIVE THE NEXT FOUR ISSUES!
PRINT & ONLINE SUBSCRIPTION
From $24.95






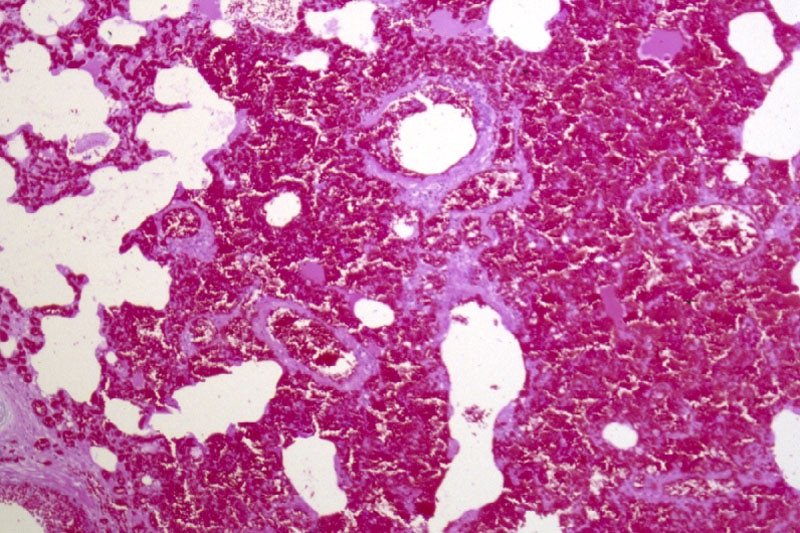


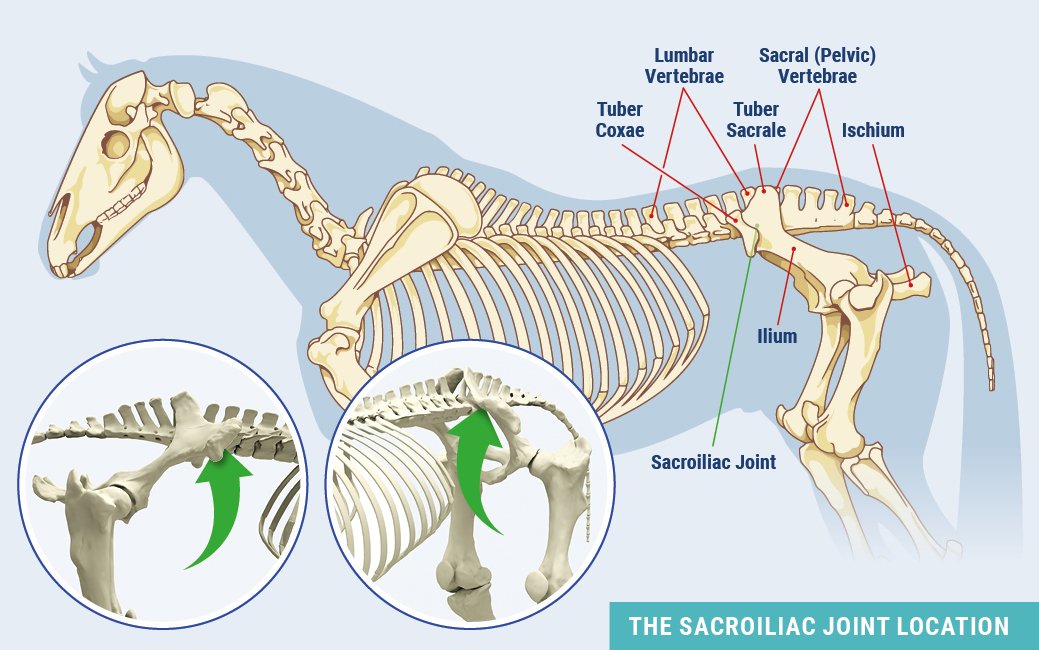
















































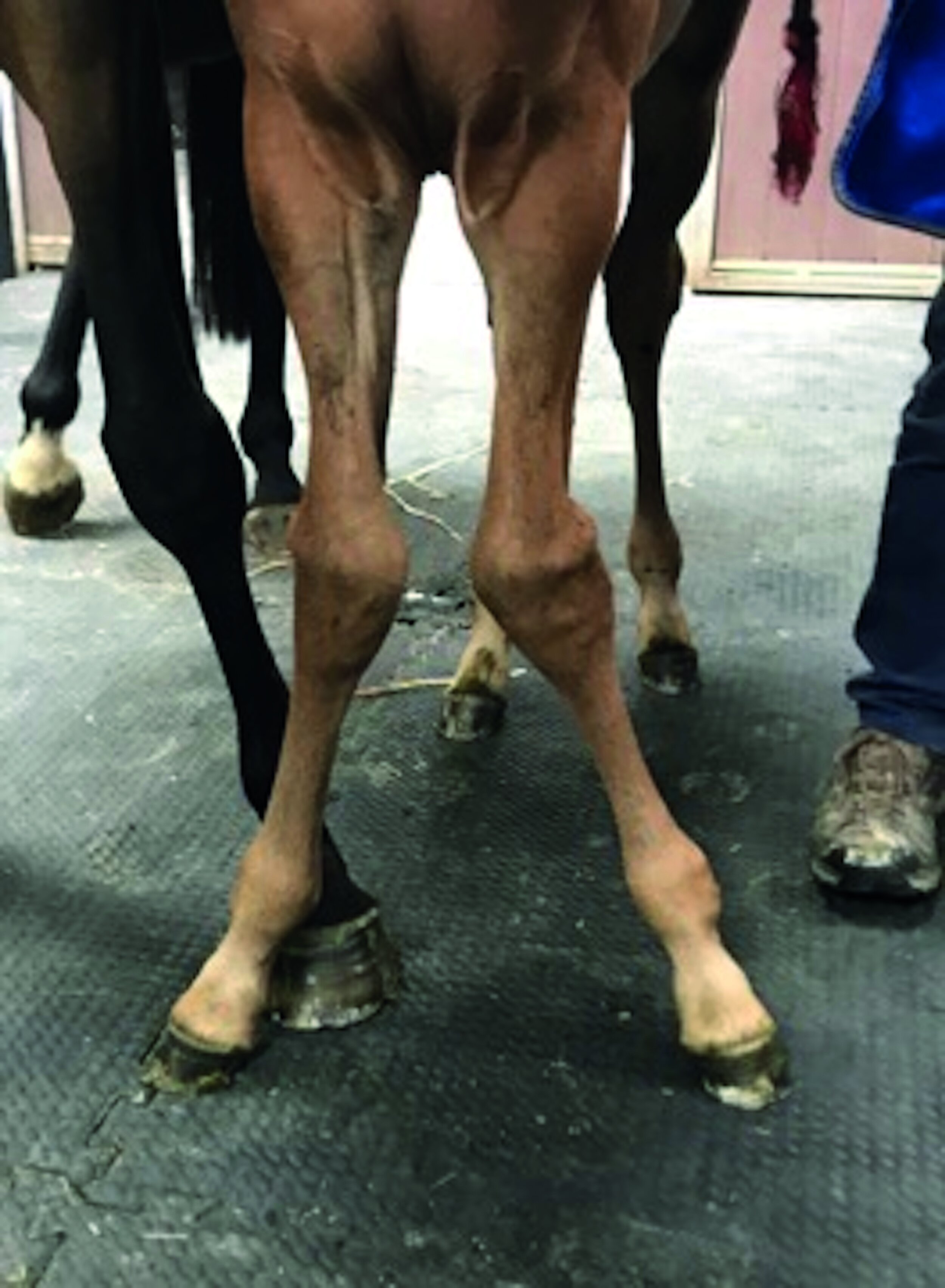


![Promising developments in quest to prevent catastrophic racehorse injuriesUniversity of Kentucky study shows association between mRNA biomarkers and catastrophic injuries in Thoroughbred racehorses—a positive step forward in the development of a pre-race screening toolCatastrophic injuries in Thoroughbred racehorses is a top-of-mind concern for the global racing industry and its fans. That sentiment is shared by researchers at the University of Kentucky and their collaborators, who are working to learn more about changes happening at a cellular level that might indicate an injury is lurking before it becomes career or life ending. Could it be possible to identify an early marker or signal in horses at risk of catastrophic injury, allowing for intervention before those injuries happen? And, if so, might this type of detection system be one that could be implemented cost effectively on a large scale?According to Allen Page, DVM, PhD, staff scientist and veterinarian at UK’s Gluck Equine Research Center, the short answer to both questions is that it looks promising. To date, attempts to identify useful biomarkers for early injury detection have been largely unsuccessful. However, the use of a different biomarker technology, which quantifies messenger RNA (mRNA), was able to identify 76% of those horses at risk for a catastrophic injury. An abstract of this research was recently presented at the American Association of Equine Practitioners’ annual meeting in December 2020 and the full study published January 12 in the Equine Veterinary Journal (https://beva.onlinelibrary.wiley.com/journal/20423306). In this initial research—which looked at 21 different mRNA markers selected for their roles in encoding proteins associated with inflammation, bone repair and remodeling, tissue repair and general response to injury—three markers showed a large difference in mRNA levels between injured and non-injured horses. For almost four years, Page and his University of Kentucky colleagues have been analyzing blood samples from almost 700 Thoroughbred racehorses. The samples, collected by participating racing jurisdictions from across the United States, have come from both catastrophically injured and non-injured horses in a quest to better understand changes that might be happening at the mRNA level and if there are any red flags which consistently differentiate horses that suffer a catastrophic injury.According to Page, the ultimate hope is to develop a screening tool that can be used pre-race to identify horses at increased risk for injury. The results of this study, which was entirely funded by the Kentucky Horse Racing Commission’s Equine Drug Research Council, suggest that analysis of messenger RNA expression could be an economical, effective and non-invasive way to identify individual racehorses at risk for catastrophic injury.Joining Page in the research from UK’s Gluck Center are Emma Adam, BVetMed, PhD, DACVIM, DACVS, assistant professor, research and industry liaison, and David Horohov, PhD, chair of the Department of Veterinary Science, director of the Gluck Center and Jes E. and Clementine M. Schlaikjer Endowed Chair.Previous research has shown that many catastrophic injuries occur in limbs with underlying and pre-existing damage, leading to the theory that these injuries occur when damage accumulation exceeds the healing capacity of the affected bones over time. Since many of these injuries have underlying damage, it is likely that there are molecular markers of this that can be detected prior to an injury.The identification of protein biomarkers for these types of injuries has been explored in previous research, albeit with limited success. The focus of this project, measuring messenger RNA, had not yet been explored, however. The overall objective was to determine if horses that had experienced a catastrophic injury during racing would show increased inflammatory mRNA expression at the time of their injury when compared to similar horses who were not injured.The genetic acronyms: A primer on DNA, RNA, mRNA and PCRThis research leverages advances made in genetics during the last several decades, both in a greater understanding of the field as well as in applying that knowledge to specific issues facing the equine industry, including catastrophic breakdown in racehorses.The genetic code of life is made up of genes and regulatory elements encoded by DNA, or deoxyribonucleic acid, which is found in the nucleus of cells in all living organisms. It is arranged in a double helix structure, similar to a twisted ladder. The rungs of that ladder are nucleotide base pairs, and the ordering of those base pairs results in the specific genetic code called a gene. The genetic code in the genes and the DNA tell the body how to make proteins. RNA (ribonucleic acid) is created by RNA polymerases, which read a section of DNA and convert it into a single strand of RNA in a process called transcription. While all types of RNA are involved in building proteins, mRNA is the one that actually acts as the messenger because it is the one with the instructions for the protein, which is created via a process called translation. In translation, mRNA bonds with a ribosome, which will read the mRNA’s sequence. The ribosome then uses the mRNA sequence as a blueprint in determining which amino acids are needed and in what order. Amino acids function as the building blocks of protein (initially referred to as a polypeptide). Messenger RNA sequences are read as a triplet code where three nucleotides dictate a specific amino acid. After the entire polypeptide chain has been created and released by the ribosome, it will undergo folding based on interactions between the amino acids and become a fully functioning protein. While looking at inflammation often involves measuring proteins, Page and his collaborators opted to focus on mRNA due to the limited availability of reagents available to measure horse proteins and concerns about how limited the scope of that research focus would be. Focusing on mRNA expression, however, is not without issues. According to Page, mRNA can be extremely difficult to work with. “A normal blood sample from a horse requires a collection tube that every veterinarian has with them. Unfortunately, we cannot use those tubes because mRNA is rapidly broken down once cells in tubes begin to die. Luckily, there are commercially-available blood tubes that are designed solely for the collection of mRNA,” he said.“One of the early concerns people had about this project when we talked with them was whether we were going to try to link catastrophic injuries to the presence or absence of certain genes and familial lines. Not only was that not a goal of the study, [but] the samples we obtain make that impossible,” Page said. “Likewise for testing study samples for performance enhancing drugs. The tubes do an excellent job of stabilizing mRNA at the expense of everything else in the blood sample.”In order to examine mRNA levels, the project relied heavily on the ability to amplify protein-encoding genes using a technique called the Polymerase Chain Reaction (PCR). By using a variety of techniques, samples from the project were first converted back to DNA, which is significantly more stable than mRNA, and then quantified using a specialized machine that is able to determine the relative amount of mRNA initially present in the individual samples. While it is easy to take for granted the abilities of PCR, this Nobel Prize winning discovery has forever changed the face of science and has enabled countless advances in diagnostic testing, including those used in this study.The research into mRNA biomarkers Catastrophic racing and training injuries have long been a target for researchers due to the high societal and welfare impacts on the racing industry. With the nearly universal requirement for necropsies on horses that succumb to these injuries, work by researchers has demonstrated that most horses with catastrophic injuries have pre-existing damage in their legs. This pre-existing damage presents an opportunity to detect injuries before they occur, whether that be with advanced imaging or less invasive techniques, such as screening of blood for injury biomarkers. Horses eligible for inclusion in the study were Thoroughbreds entered into any race in one of five participating jurisdictions from September 2017 to June 2020. To look at the mRNA, these jurisdictions collected specific blood samples either pre-race or post-race from a selection of non-injured horses or immediately from a horse after a catastrophic injury. Once collected, samples were sent to the Gluck Center where they were analyzed using PCR. The names of horses and sample types (injured, pre-race or post-race) were kept from the researchers until the samples had been fully analyzed.Once the names and dates of samples were revealed, public records were then used to learn more about each horse. Information examined included the horse’s sex, age, race type and whether non-injured horses raced again within three months of the sampled race. For horses who had been catastrophically injured, necropsy results were used to categorize the type of musculoskeletal injury that occurred. “Out of the 21 markers (genes) that were measured, three of them immediately stood out as being able to predict injury. The three individual markers of interest were Insulin-like Growth Factor 1 (IGF-1), Matrix Metalloproteinase-2 (MMP2) and IL-1 Receptor Antagonist (IL1RN). Taken together, the changes seen in all three of these markers suggest that there is increased inflammation in the injured horses and that the inflammation arises from bone, just as was suspected,” Page said.“Based only on these three markers, we were able to correctly identify horses at risk for injury 76% of the time and exclude horses for being at risk 88% of the time,” Page said. “Obviously, we want to maximize those numbers as much as possible, so while there’s room for improvement, this is significantly better than any other option currently available.”One of the limitations of the study was that horses were only sampled once, so there was no ability to identify changes in individual horses over a period of time. Once horses start being sampled repeatedly on a regular basis with this testing, Page said he believes the ability to identify at-risk horses will improve dramatically.What does the future hold?“Since the ultimate hope is to develop a commercially-viable screening tool that can be used pre-race to identify horses at increased risk for injury, we anticipate adding multiple other markers with a new study that is just getting started,” Page said.As part of the new study, also funded by the Kentucky Horse Racing Commission, Page and two Gluck Center colleagues, James MacLeod, VMD, PhD, John S. and Elizabeth A. Knight chair and director of UK Ag Equine Programs, and Ted Kalbfleisch, PhD, associate professor, plan to utilize RNA-sequencing, a relatively new technology, to expand their search to all of the approximately 22,000 protein-coding genes horses have. This will dramatically increase the likelihood that they will be able to identify additional markers for horses at risk of injury. They plan to do this by using the large number of samples that have already been collected, further leveraging their initial research and decreasing the amount of time it will take to complete their new study.“We are really excited about this new project and the promise that it holds,” Page said. “In our first study, we drove the data because we had to select which mRNA markers we wanted to examine. In our new study, the RNA-sequencing data is really what will be driving us.”While that project is ongoing, Page and his colleagues continue to refine and improve upon the various laboratory steps required to isolate and analyze mRNA. Guided by the hope of providing the racing industry with a high-throughput screening tool, the group has employed multiple robotic platforms that can already handle 100 samples per day and be easily scaled up to handle more.“As a researcher, I see it as being my job to provide practical and reliable solutions to the horse racing industry,” Page said. “I know that change can be scary, but we can all agree that something needs to change to help better protect racehorses and the jockeys who ride them. Ultimately, the racing industry will decide when it wants to give this screening tool a chance. I’m confident that, when the industry is ready, we will be too.” The full study published in the Equine Veterinary Journal can be found here: https://doi.org/10.1111/evj.13423](https://images.squarespace-cdn.com/content/v1/517636f8e4b0cb4f8c8697ba/1619181103562-L9SRTHKK0L6C0FFYFXA2/Screenshot+2021-04-23+at+13.31.27.png)